Samuel Yee War Leung1, Diya Baker2, Nicole Leung3
1Foundation Year 2 Doctor, Royal Shrewsbury Hospital, Shropshire, West Midlands
2Ophthalmology Specialist Registrar Year 1, Royal Shrewsbury Hospital, Shropshire, West Midlands
3Band 8a Pharmacist, Specialist Interest in Ophthalmology
Learning points
- Moraxella Non-liquefaciens is a cause of corneal ulcers that are known to have poor clinical outcomes
- Our experiences and previous case series suggest a prolonged time until epithelisation
- Prolonged clinical course with poor outcomes
- Moraxella Non-liquefaciens is a commensal in the upper respiratory tract but is a very unusual cause of ocular pathologies and we have hypothesised that redirected exhaled air as a possible route of infection
Introduction
Mask wearing may increase the risk of ocular infections with organisms that are not usually encountered as pathogenic. Moraxella Non-liquefaciens is normally commensal in the upper respiratory tract, non-pathogenic, and rarely found to be the causative organism in microbial keratitis (1). At the Ophthalmology department covering the England West Midlands North we encountered 4 patients, each with a unique Moraxella strain after the end of the second lockdown.
| Case | Age | Sex | Diagnosis | Epilisation Time | Presenting VA | Final VA |
|---|---|---|---|---|---|---|
| 1 | 69 | F | Bacterial corneal ulcer | 62 days | HM | 6/18 |
| 2 | 89 | F | Bacterial corneal ulcer | Lost to Follow-up | – | – |
| 3 | 74 | F | Bacterial corneal ulcer | 72 Days | 6/24 | 6/7.5 |
| 4 | 71 | F | Bacterial corneal ulcer | 12 Days | CF | CF |
Microbial Keratitis (MK) is a corneal infection which can lead to permanent vision loss if corneal scarring or perforation occurs, its heavily associated with contact lens wear. It is reported to account for 1 million health care visits in the US annually, of which 70000 are confirmed cases (2-4). The most common organisms isolated in MK are Pseudomonas, Staphylococcus aureus, and streptococci (5).
We presented a case for an elderly woman who was one of four patients presenting within a month, with a corneal ulcer. Each patient had grown a species of Moraxella Non-liquefaciens unique to the individual, dispelling the concern that the results were contaminated. Not one of these patients used contact lenses. Since the Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic there has been long-term use of face masks in the public(6,7). Research states that face masks are associated with both ocular irritation and dryness and increased rates of ocular infection(7,8).
Findings
A 69-year-old female was referred to the Eye Department by the GP with concerns of a painful, discharging right eye. She reported no significant ophthalmic history, before this. Pain in the right eye began in early December 2021 before developing into purulent discharge by late December. No further symptoms were reported systemically, and no obvious focal neurology was noted on examination.
On initial presentation, her pin hole visual acuity (PHVA) was Hand Movements. Slit lamp examination revealed a Corneal ulcer with hypopyon measuring 1.5mm. Based on a working diagnosis of microbial keratitis, corneal scrapes were sent to the microbiology department for culture and sensitivity screen alongside a viral PCR for HSV. Cefuroxime 5% eyedrops, Exocin (ofloxacin) 0.3% eyedrops were started hourly. A Gram-Negative Bacilli reportedly grew on the agar plates.
Four days into presentation the corneal ulcer increased to 3.5mm, with franker uptake of fluorescein stain and dense exudates on the endothelium. A unique Moraxella non-liquefaciens was identified. Gentamicin 1.5% was added hourly in the daytime only alongside Chloramphenicol ointment 1% at night and oral Doxycycline 100mg twice a day for 2 weeks. Ascorbic Acid 2g once a day was also added to aid healing of the corneal tissue.
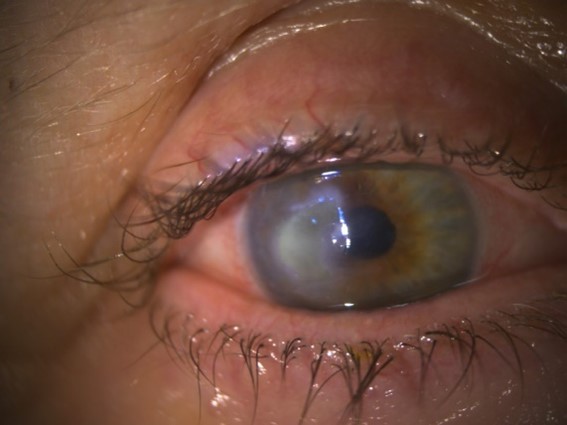

Eight days post initial presentation, acyclovir was started in order to treat a potential underlying herpetic disease and dexamethasone 0.1% eye drops was started on a tapering regime to reduce inflammation. There was no visible improvement in the appearance of the cornea but PHVA was now 1/60. Gentamicin and Cefuroxime was stopped, Ofloxacin 0.3% reduced to five times a day and chloramphenicol were continued at once a night.
Nineteen days into presentation, whilst PHVA remained at 1/60, the appearance of the right eye improved significantly with a visibly smaller hypopyon and reduced activity in the anterior chamber. On the other hand, the epithelial defect still measured 3.5mm located temporal to the visual axis.
Sixty-two days into presentation, the appearance of the corneal ulcer looks to have stabilised with no changes to the size, and still showed signs of underlying infiltrate, PHVA was reported as 6/18. Management was further optimised to directly replace Ofloxacin 0.3% with Chloramphenicol (PF) 0.5% minims 6 times daily, Aciclovir reduced to 400mg twice daily for 5 days and Dexamethasone tapered until stop.
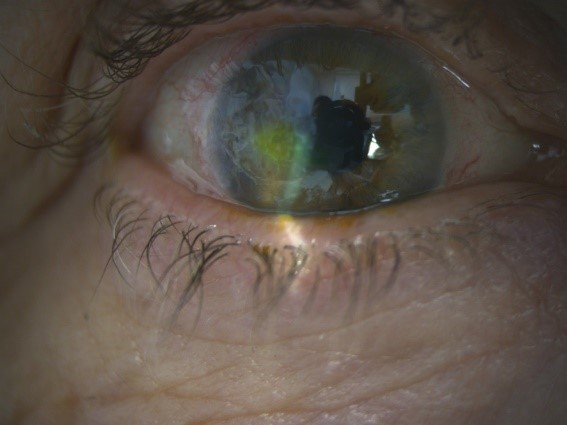
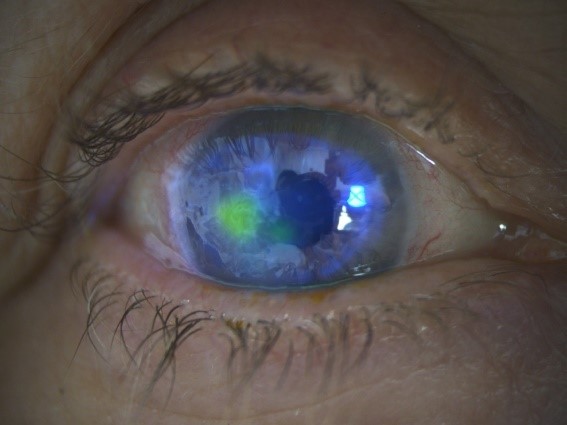
Discussion
Microbial keratitis is linked to several pre-disposing risk factors including contact lens wear and ocular surface disease(9). The three main species identified as causative organisms are Pseudomonas aeruginosa, Staphylococcus and Streptococcus (9). Keratitis due to Moraxella species is not common, the prevalence is low accounting only for 3-5% of all bacterial ulcers, with a reported seasonal component of higher incidence in colder temperatures (9,10). Normally, Moraxella non-liquefaciens is considered a part of normal flora in the upper respiratory tract and generally pathogenicity is low (11). Unless at extremes of age, such as children/elderly where more cases of localised Moraxella infections have been reported, which was reasoned to be related to a compromised host immunity such as co-existing diabetes(11). The visual prognosis of contracting this organism is still one of the worst compared to other infective species (12). The infection is very slow to respond to antimicrobial therapy and has a poor visual outcome, the risk of blindness can occur in one-third of affected patients (12).
Moraxella nonliquefaciens carries the worst prognosis of the Moraxella species that can be isolated from the eye (13,14). The issue is that time taken to heal from such an infection can be very slow due to persistent inflammation of the cornea (15).Treatment up to 68 days had been reported in other case series, using aminoglycosides, fluoroquinolones noting changes up to complete resolution of corneal epithelial defects and stromal infiltration(9,16). Agents such as chloramphenicol, ofloxacin and cefuroxime are very effective against the species as a whole (12).
Whilst contact lens remain the most associated risk factor for microbial keratitis, it was not applicable for this patient. One possible explanation of this high incidence of Moraxella ocular infections could have been use of face masks which had potentially promoted infection. The redirection of exhaled air may have increased the exposure of the cornea’s surface to Moraxella non-liqufaciens (17,18). The implication of redirected exhaled air is linked to mask-associated dry eye (MADE) which has since become more prevalent during the pandemic(19). A study using thermal imaging demonstrated this leakage of breathing air to the eye whilst wearing a standard issue surgical facemask(17). The significance is that dry eyes will disrupt a healthy tear film, removing the associated antimicrobial properties thus increasing a patient’s susceptibility to infections by impairing the eye’s innate immunity (20). A diverse ocular microbiota will also compete against and prevent pathogenic species from colonisation (21).
Conclusion
With mask usage becoming a staple of European attire, there is a possibility of this increasing the incidence of infections from organisms that are not usually encountered as pathogenic, particularly microbes disseminating within exhaled air. In this case, the impact of such pathogens led to a significant change in the patient’s visual function and acuity. Therefore, such the findings need to be considered with the utmost diligence. Despite no causality being shown in this case report, this hypothesis will require further research to add credibility or deny the claim.
This unusual pathogen encounter highlights the importance of early diagnosis and treatment with empirical antibiotics can result in a positive visual outcome and as such there is scope for continuing with current standard local/national guidance. Overall, with the possibility of a growth in this trend, there needs to be a propensity to modulate these guidelines to consider rarer causes of keratitis as well as, highlighting this to local units.
References
1. Porcar Plana CA, Matarredona Muñoz J, Moya Roca J, Campos Mollo E. Moraxella nonliquefaciens superinfecting herpes simplex keratitis. Eur J Ophthalmol 2021:11206721211019565.
2. Collier SA, Gronostaj MP, MacGurn AK, Cope JR, Awsumb KL, Yoder JS, et al. Estimated burden of keratitis–United States, 2010. MMWR Morb Mortal Wkly Rep 2014 Nov 14;63(45):1027-1030.
3. Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology 2017;124(11):1678-1689.
4). Lin A, Rhee MK, Akpek EK, Amescua G, Farid M, Garcia-Ferrer FJ, et al. Bacterial keratitis preferred practice pattern®. Ophthalmology 2019;126(1):P1-P55.
5. Bennett L, Hsu HY, Tai S, Ernst B, Schmidt EJ, Parihar R, et al. Contact Lens Versus Non–Contact Lens–Related Corneal Ulcers at an Academic Center. Eye & Contact Lens 2019;45(5):301-305.
6. Reichart S, Behrens-Baumann WJ, Dirschmid H, Gallastroni LP, Mennel S. Severe Acute Respiratory Syndrome Coronavirus 2, Gesichtsmaske und Keratitis. Der Ophthalmologe 2021;118(7):707-709.
7. Tang YF, Chong EW. Face Mask–Associated Recurrent Corneal Erosion Syndrome and Corneal Infection. Eye & contact lens 2021;47(10):573-574.
8. Moshirfar M, West WB, Marx DP. Face mask-associated ocular irritation and dryness. Ophthalmology and therapy 2020;9(3):397-400.
9. Kenny SE, Puig M, Salinas R, Johnson DA, Kheirkhah A. Moraxella Keratitis: A Case Series. Eye & contact lens 2021;47(12):674-676.
10. Hoarau G, Merabet L, Brignole-Baudouin F, Mizrahi A, Borderie V, Bouheraoua N. Moraxella keratitis: epidemiology and outcomes. European Journal of Clinical Microbiology & Infectious Diseases 2020;39(12):2317-2325.
11. Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science 2009;326(5960):1694-1697.
12. McSwiney TJ, Knowles SJ, Murphy CC. Clinical and microbiological characteristics of Moraxella keratitis. Br J Ophthalmol 2019;103(12):1704-1709.
13. Takahashi S, Murata K, Ozawa K, Yamada H, Kawakami H, Nakayama A, et al. Moraxella species: infectious microbes identified by use of time-of-flight mass spectrometry. Jpn J Ophthalmol 2019;63(4):328-336.
14. Yi H, Yong D, Lee K, Cho Y, Chun J. Profiling bacterial community in upper respiratory tracts. BMC infectious diseases 2014;14(1):1-10.
15. Inoue H, Suzuki T, Inoue T, Hattori T, Nejima R, Todokoro D, et al. Clinical characteristics and bacteriological profile of Moraxella keratitis. Cornea 2015;34(9):1105-1109.
16. LaCroce SJ, Wilson MN, Romanowski JE, Newman JD, Jhanji V, Shanks RM, et al. Moraxella nonliquefaciens and M. osloensis are important Moraxella species that cause ocular infections. Microorganisms 2019;7(6):163.
17. Hadayer A, Zahavi A, Livny E, Gal-Or O, Gershoni A, Mimouni K, et al. Patients wearing face masks during intravitreal injections may be at a higher risk of endophthalmitis. Retina 2020;40(9):1651-1656.
18. Blom K, Bragadottir R, Sivertsen MS, Moe MC, Jorstad OK. Mask use by patients in the context of COVID-19 can increase the risk of postinjection endophthalmitis. Acta Ophthalmol 2021 Jun 21.
19. Boccardo L. Self-reported symptoms of mask-associated dry eye: A survey study of 3,605 people. Contact Lens and Anterior Eye 2021:101408.(20) McDermott AM. Antimicrobial compounds in tears. Exp Eye Res 2013;117:53-61.
21. Petrillo F, Pignataro D, Lavano MA, Santella B, Folliero V, Zannella C, et al. Current evidence on the ocular surface microbiota and related diseases. Microorganisms 2020;8(7):1033.
