Raheel Faiz1, Samer Elsherbiny2
1Foundation Year 2 Doctor, University Hospitals Coventry & Warwickshire NHS Trust, Coventry, United Kingdom
2Consultant Ophthalmologist, South Warwickshire Foundation Trust, Warwickshire, United Kingdom.
Abstract
Background
Retinal vein occlusion is associated with the release of vascular endothelial growth factors (VEGF) and a number of inflammatory mediators (1). Management is aimed at treating the macular oedema and any neovascular changes. This includes the use of the licenced options of anti-VEGF injections, intravitreal dexamethasone and retinal laser photocoagulation.
Case presentation
We present a case with a sustained 2-year effect post one FA implant. A 72-year-old male patient presented with a left hemi-retinal vein occlusion with macular oedema in January 2013. Initial treatment was intravitreal dexamethasone implants. Although he showed an excellent response, it became short lived with further retreatment, with the effect lasting for 8-10 weeks on average before oedema returned. This necessitated additional treatment with numerous anti-VEGF injections in addition to treating angiographically ischemic areas with retinal laser. At 17 months follow up, after off label use of FA, we demonstrated an improvement in both visual acuity and central subfield thickness for the patient:
- Visual acuity: 6/30 before the FA implant, to 6/15 at 26 months after implant.
- Central subfield thickness (CST): at baseline: 477μm; maximum pre-FA: 555μm; immediately pre-FA: 447μm; at 26 months post-FA: 304 μm.
Conclusion
Retinal vein occlusion is a common pathology that can be treated by various means. The off label fluocinolone acetonide (FA) implant demonstrated a sustained response over 2 years in this case. Further prospective studies of its use are needed to confirm its role in clinical practice.
Introduction
In central retinal vein occlusion (CRVO), extravasation of blood into the retina gives rise to macular oedema which is further exacerbated by the pathophysiological release of VEGF. Management by an ophthalmologist is often aimed at treating the macular oedema and includes intravitreal steroid implants and/or anti- VEGF intravitreal injections. Laser photocoagulation is also used to treat ischaemic changes in the retina and in the presence of retinal, optic disc or iris neovascularization.
More recently, limited evidence suggests fluocinolone acetonide intravitreal implant can be used to treat macular oedema that arises from RVO.
Ramchandran et al (2008), (2) reported the 12 month results of a prospective, noncomparative, interventional case series for the use off label fluocinolone acetonide (FA) in patients with central retinal vein occlusion. This was associated with the statistically significant improvement in vision and central retinal thickness. Unpublished data by Ribeiro et al, (2021) (3) reported similar findings at 12 months but included one case of CRVO and one of branch RVO (BRVO). The emphasis was essentially on the use of FA as monotherapy for CRVO and BRVO when there had been good but short-lived response to the use of Ozurdex (the biodegradable steroid implant).
Case report
This is a case report of a 72 year-old male who first presented with hemi-retinal vein occlusion with macular oedema in January 2013 and underwent implantation of fluocinolone acetate (FA) implant in April 2020. We present our findings 2-years post-Iluvien implant. Previously 14-month follow-up demonstrated improvements in visual acuity (VA), from 6/30 to 6/12 (with pinhole), and optical coherence tomography (OCT) parameters; central subfield thickness 477 μm to 311 μm. The two licensed anti-VEGF treatment options available: Eylea (aflibercept) and Lucentis (ranibizumab) intravitreal injections (IVI) to treat his macular oedema were given between 2013-2019. During this time, he had fourteen Ozurdex (dexamethasone) implants showing very good response in terms of VA of 6/24 to 6/6, achieved on three occasions until September 2018, when the initial treatment benefits became short-lived, with macular oedema returning after 8-10 weeks on average. Based on his best response having been to Ozurdex, FA was proposed and once funding for FA (Iluvien) implant was approved, and he underwent this in April 2020. 2 years post FA implant, he has had no further injections since the FA implant. At clinic follow-up no IOP increase or side effects were detected.
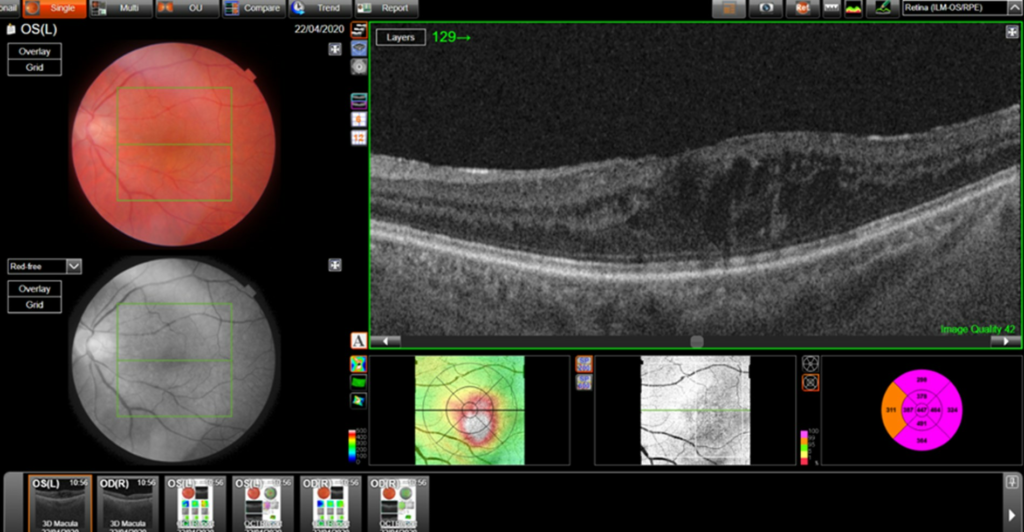
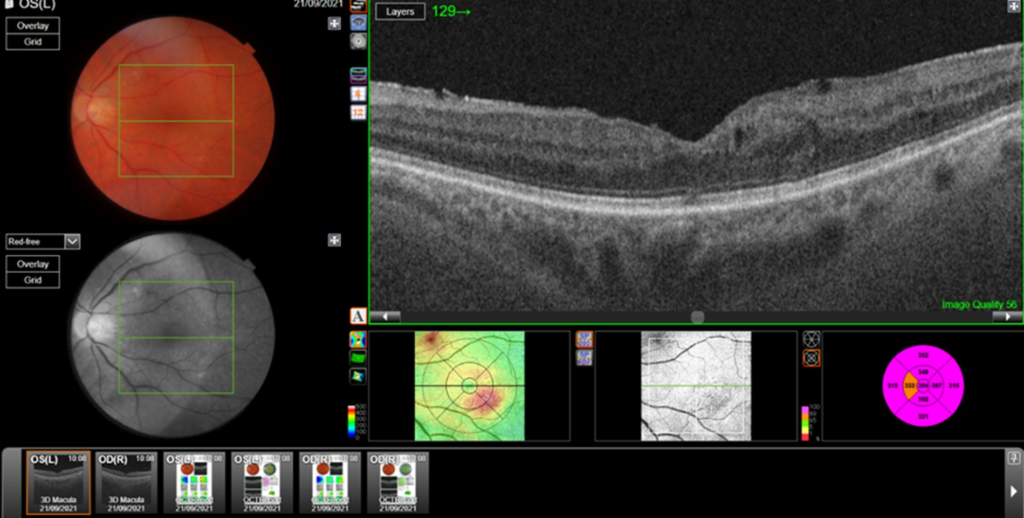
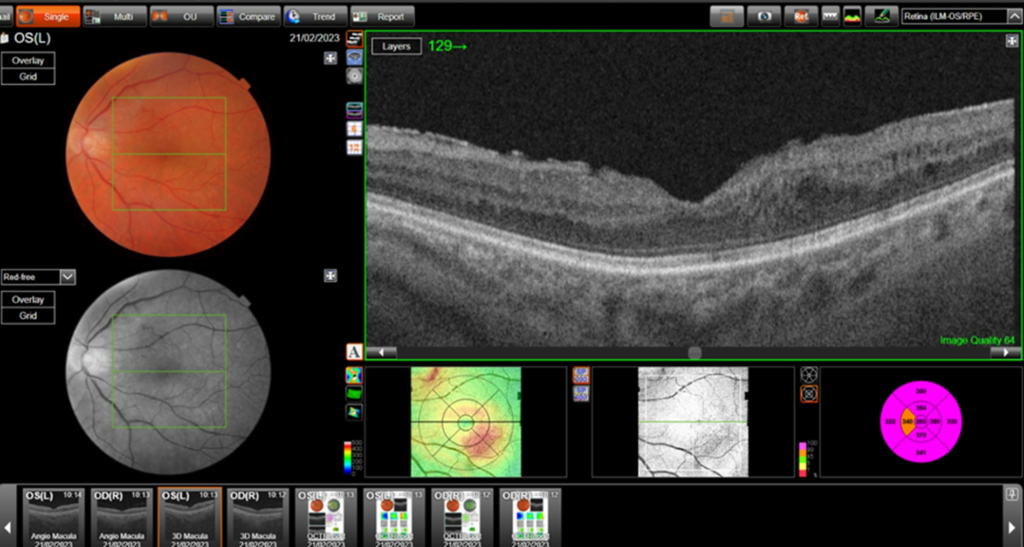
Central retinal thickness = 477 μm (baseline); 555 μm (maximum pre-FA);
Discussion
Use of anti-VEGF and steroids is well established in CRVO and macular oedema. Monotherapy tends to be effective, however the role of combination therapies is not well established and there is no consensus in current published guidelines or literature regarding this (4).
Combination of retinal laser therapy, anti-VEGF and steroids can be used to treat angiographically ischaemic areas, with no studies showing superiority of either one of these three modalities. Ramchandran et al have demonstrated that the use of fluocinolone acetonide for the first 12 months showed significant improvement in VA (from VA 20/126 pre implant to 20/80 by 12 months). The mean and median central retinal thickness reduced from 622 and 600 micrometres respectfully pre-injection to 307 and 199 micrometres post-injection respectfully.
As Ribeiro et al illustrated when comparing the use of anti-VEGF to fluocinolone acetonide implants, patients who received anti-VEGF injections required more repeat administration as well a dexamethasone implant compared to patients treated with fluocinolone acetonide where the effects were longer lasting and showed greater improvement. However, although patients receiving fluocinolone acetonide implants in this study have demonstrated increased duration of efficacy, side effects such as cataract and glaucoma including the potential need for surgical treatment were much higher.
Our patient showed good, sustained response to single FA treatment, corroborating the only other published case report, but for double the follow up period. While this indicates the possibility of FA being a satisfactory monotherapy, numerous issues around timing in the context of existing licenced options, the role of peripheral retinal laser to ischaemic areas and funding of FA implants for off-label use will all need to be addressed. This would require the appropriate large-scale, prospective studies to influence and guide standard practice. However, we maintain off label FA implants may be a viable option in the event that patients have a similar clinical course to our patient.
Conclusion
Retinal vein occlusion is a common pathology that can be treated by various means. Evidence is slowly gathering in favour of fluocinolone acetonide implants being able to improve the macular oedema that arises from this pathology without resulting in any significant side effects such as changes in IOP. Longer term, larger prospective studies are needed to address this more reliably.
References
- Pichi, F. (2022) Central retinal vein occlusion, EyeWiki. Available from: https://eyewiki.aao.org/Central_Retinal_Vein_Occlusion [Accessed May 15 2023].
- Ramchandran RS, Fekrat S, Stinnett SS, Jaffe GJ. Fluocinolone acetonide sustained drug delivery device for chronic central retinal vein occlusion: 12-month results. American Journal of Ophthalmology. 2008;146(2). doi:10.1016/j.ajo.2008.03.025
- Ribeiro M. 12-month effectiveness of the fluocinolone acetonide implant (ILUVIEN®) in retinal vein occlusions – two clinical cases [Internet]. 2021 [Accessed May 15 2023]. Available from: https://euretina.org/resource/abstract_2021_12-month-effectiveness-of-the-fluocinolone-acetonide-implant-iluvien-in-retinal-vein-occlusions-two-clinical-cases/
- Lip PL, Cikatricis P, Sarmad A, Damato EM, Chavan R, Mitra A, et al. Efficacy and timing of adjunctive therapy in the anti-VEGF treatment regimen for macular oedema in Retinal vein occlusion: 12-month real-world result. Eye. 2017;32(3):537–45. doi:10.1038/eye.2017.230
