Alice Ditchfield
Introduction
The cornea, in addition to providing immunological protection and mechanical integrity to the eye, has its most important role in vision. This is due to its transparency and ability to refract light (1). Corneal scarring, and hence loss of transparency, is therefore of major clinical importance and one of the leading causes of blindness worldwide. Consequently, our understanding of the mechanisms responsible is highly important for the development of potential treatments (2). Its anatomical position means the cornea is susceptible to damage, which results in the wound healing process (1). While in other areas of the body this will result in scar formation, this is minimised in the cornea to prevent loss of visual acuity (3). However, under certain circumstances, including infection, inflammation, disease, and the entry of debris, corneal scarring will occur (2).
Within the literature and among ophthalmologists, a term commonly used to refer to loss of transparency in the cornea is “corneal haze”. Corneal haze can be considered as “the pathologic light scattered back to an observer during an examination” (4), and will here be used to describe opacity in the cornea as a result of wound healing leading to corneal scarring.
This review aims to elucidate the key mechanisms behind the formation of corneal scars, and to give some consideration to the strength of the evidence behind those mechanisms identified. The review will firstly discuss the process of normal wound healing within the cornea that may precede scarring, and then examine three key factors within this process that have been suggested to contribute to corneal scarring: keratocytes and their activation by growth factors, collagen fibril organisation, and the role of neutrophils. Examination of the literature suggests that keratocyte activation by growth factors to their repair phenotype was found to be the most important factor in the formation of scars, while the disorganised deposition of collagen is a more contentious factor, and neutrophils have only relatively recently been thought to play a role and consequently little supporting evidence currently exists.
This knowledge has allowed the development of targeted treatments, and perhaps further progress will be made in the future as our understanding of corneal scarring mechanisms improves.
The Wound Healing Process
It is prudent to consider the wound healing process in general before examining the mechanisms that lead from this to scarring. Scarring may arise from this process in the case of certain types of injury, such as: deep abrasions, lacerations, ulcerations, acid or alkali burning, and disease such as herpes simplex, keratitis and syphilis. If dense enough, corneal scarring will lead to haze, hence affecting vision (5).
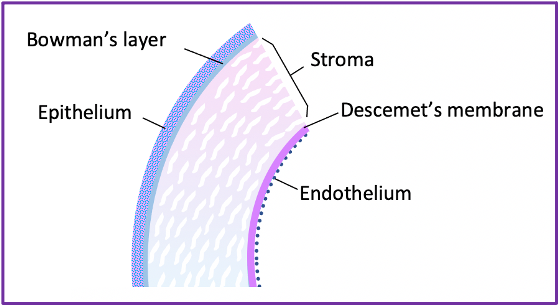
Wound healing can be split into three broad areas based on the corneal layer involved- epithelial, stromal and endothelial healing. The epithelium is the most superficial of the corneal layers, while the endothelium is the deepest (see Figure 1). Epithelial wound healing will be considered first.
The epithelium of the cornea goes through three phases of healing; first, a latent phase, for between three and six hours from when the injury occurs; next, migration and adhesion of epithelial cells to the wound site; and finally, the proliferation of epithelial cells to restore epithelial thickness (3).
During the latent phase, damaged epithelial cells undergo apoptosis (1) and are sloughed from the surface of the eye. Basal and squamous cells around the wound separate due to the loss of hemidesmosomes and tight junctions (6). A study using the epithelium from rabbit corneas showed, that within two hours of wounding, all hemidesmosomes between basal epithelial cells and the basement membrane (the Bowman’s layer) within 50-70mm from the wound edge were lost, with those up to 200mm also being significantly reduced (7). A limitation of studies such as this, that use rabbit corneas to model humans’, is that there are some significant anatomical differences that may produce differences in wound healing mechanisms, such as the lack of a Bowman’s layer in rabbit corneas.
However, as this study concerns the epithelium alone, and used a straightforward and accurate method of analysis to produce the results, it is still a valuable source of evidence for the mechanism of human epithelium healing.
The epithelial cells surrounding the wound then begin to develop finger-shaped projections called filopodia, and wider lamellipodia (see Figure 2) (3).
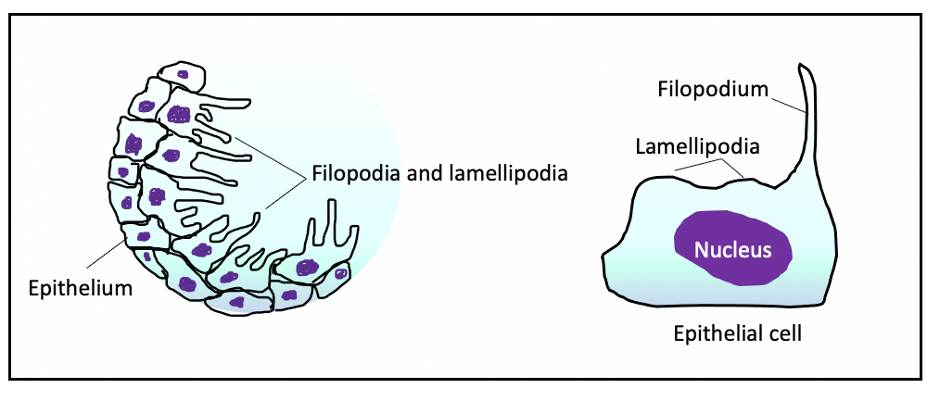
For epithelial cell migration and adhesion to occur, the extension of the filopodia and lamellipodia is completed. Temporary adhesions form between the filopodial and lamellipodial extensions and the underlying matrix, allowing the cells to slide towards and onto the wound (3). Adjacent cells retain their desmosomal attachments to one another, so their relative positions are maintained. They migrate as sheets of cells onto the wound surface (6), until an epithelial monolayer has formed. This may take 24 to 36 hours (3).
The final stage in epithelial wound healing is proliferation of epithelial cells. The monolayer of cells that has covered the wound surface is formed from basal cells (1), which become known as transient amplifying cells (TACs) as they undergo mitosis. The new cells which are formed are known as post-mitotic cells (PMCs), which finally form terminally differentiated cells (TDCs). Both the PMC and TDC cells types are considered suprabasal cells that overlie the basal epithelial cells. These cells move to the central area of the wound site and upwards, until the normal epithelial thickness is restored. Epithelial wound healing is considered completed when these new cells form further hemidesmosomes, allowing permanent adhesion to the underlying stroma (3).
The next area of corneal healing that will be considered is stromal healing. Repair of the stroma is critical for ensuring restoration of transparency after injury (1). The stroma underlies the epithelium and is superior to the endothelium (see Figure 1) (3), and consists largely of collagen fibrils (lamellae), produced by keratocytes, which also reside in the stroma. The keratocytes usually exist in a mitotically quiescent state, with a slow turnover rate (two to three years), responsible for producing and maintaining the extracellular matrix (ECM).
Typically, the first aspect of stromal repair after injury involves apoptosis of a proportion of the stromal keratocytes; meanwhile, other keratocytes are metabolically transformed into repair phenotypes, fibroblasts and/or myofibroblasts (8). This process is mediated by transforming growth factor beta (TGFb) and platelet-derived growth factor (PDGF) (3), and is activated by stromal and epithelial damage (9). Fibroblasts and myofibroblasts both contribute to normal wound healing by producing new ECM through the synthesis of collagens, proteoglycans and glycoproteins (3). Myofibroblasts, however, are more important in producing opacity as they produce ECM components at a more rapid rate, and generate the strength required to close the wound by regulating contractile elements (8).
Once the overlying epithelial basement membrane has been re-established, TGFb and PDGF levels will fall, the active myofibroblasts will undergo apoptosis, and the keratocyte population will be restored (3).
The final part of corneal healing to consider is endothelial healing. The endothelium underlies the stroma, with the Descemet’s layer forming its basement membrane (10). Its integrity is critical for transparency, as it draws excess fluid out of the stroma to maintain a dehydrated state, essential for stromal clarity.
If the endothelium is damaged, repair occurs by migration of adjacent cells to the site of the wound, by sliding over the Descemet’s layer or a temporary fibronectin matrix (3). This is accompanied by enlargement of the endothelial cells to increase their surface area and cover the damage; there is no mitosis of human endothelial cells, unlike in rabbits. The endothelial cells will also secrete materials to form new Descemet’s membrane if this is breached. Normal function usually returns to the corneal endothelium within a few days of damage (10).
Now that the processes of wound healing have been discussed, the mechanisms that may lead from this to corneal scarring can be examined in depth.
Keratocytes and Activation by Growth Factors
As discussed previously, damage to the cornea will result in the activation of stromal keratocytes to produce myofibroblasts, which act to remodel the stromal ECM. The key growth factors responsible for keratocyte activation are TGFb and PDGF.
In certain circumstances, this process may lead to corneal haze; myofibroblasts have a greater light-scattering effect than quiescent keratocytes, hence the resultant opacity when myofibroblasts are high in number. This effect on light is produced by the nucleus, cell body and dendritic processes of the myofibroblasts (11).
Due to this, the prolonged presence of myofibroblasts in the place of keratocytes is more likely to result in hazing. This can occur when there is a delay in the repair of the epithelial basement membrane (EBM), which allows TGFb and PDGF to continue to enter the stroma and perpetuate the raised myofibroblast population.
In addition, the presence of myofibroblasts slows the restoration of the quiescent keratocyte population, which is necessary for EBM repair. Until EBM repair occurs, TGFb and PDGF will continue to pass into the stroma and generate high levels of myofibroblasts. Only when the EBM is fully restored will TGFb and PDGF levels fall, hence inducing myofibroblast apoptosis and keratocyte repopulation (1).
In this way, EBM integrity and myofibroblast population are critically linked and may be responsible for corneal haze when influencing one another.
If the EBM is fully re-established, transparency may be restored to the cornea. However, prevention of EBM repair may occur due to recurrent corneal erosions, Alport syndrome, lattice corneal dystrophy, or thin basement membrane disease(12). In these instances, corneal haze may be a consequence.
The growth factors which transform the keratocytes to myofibroblasts therefore also play a role in hazing, through mediating this transformation. While TGFb and PDGF have been identified as the key growth factors responsible, a study conducted in 2008 suggests that epidermal growth factor (EGF) works synergistically with TGFb1 to transform keratocytes to their repair myofibroblasts.
EGF can be used to apply topically to corneal incisions, as it is effective at bringing about wound closure by increasing tensile strength, and stimulates proliferation of corneal epithelial cells. The study suggests, however, that EGF may cause perpetuation of the presence of the myofibroblasts, and in doing so contribute to the production of haze (8).
The study investigated the effects of EGF on stromal keratocyte proliferation, differentiation and expression of ECM components, by using thin layers of stroma from rabbit eyes, and extracting the keratocytes from these eyes. The rabbit corneal keratocytes were incubated with EGF, TGFb1, and a combination of EGF and TGFb1, for three days. The results showed that while TGFb1 alone caused 12% of keratocytes to differentiate to myofibroblasts, EGF combined with TGFb1 stimulated 90% of cells to differentiate, and produced a significant increase in the expression of fibronectin. These results offer an explanation as to why transparency is reduced in corneal wounds treated with EGF, and therefore suggest that EGF is in part responsible for hazing (8).
With regards to the usefulness of this study, it also poses the aforementioned issue regarding anatomical differences between human and rabbit corneas, which may limit the applicability of the findings to human physiology. The investigation, however, involves isolated keratocytes rather than those residing within the stroma, which may increase the confidence we can have in its applicability as there unlikely to be influence from surrounding anatomy. Furthermore, keratocytes were treated with TFGb1 and EGF alone to act as comparators, and the results were tested by also carrying out the procedure with inhibited EGF receptors, providing more information on the mechanism involved and hence increasing the study’s usefulness. As the study was carried out in 2008, it is likely that the technology was sufficiently up to date, and the methods, which have been clearly outlined, were appropriate, making it an authoritative source.
Research has also identified other growth factors such as connective tissue growth factor (CTGF) as contributors to the formation of corneal haze (13). There is a very strong evidence base behind the role of myofibroblasts in the healing cornea in producing haze, and the indirect contribution of growth factors is also well documented. This knowledge may be used to develop targeted treatments of haze in the future; a study has shown that anti-TGFb neutralising antibodies can inhibit the process of keratocyte differentiation (14). There is also evidence to suggest that stimulation of the healing cornea with TGFb3 may also reduce scarring as it stimulates different pathways from TGFb1 and TGFb2 (15). This knowledge may eventually be used clinically to prevent corneal scarring.
Collagen Fibril Organisation
Collagen fibrils arranged in lamellae are the predominant component of the corneal stroma. After corneal injury, activated stromal repair keratocytes increase the rate of production of ECM, including collagen, to carry out stromal remodelling.
It is suggested by many sources that the highly regular interfibrillar spacing and uniform fibril diameter in normal corneas play an important role in ensuring transparency. Fibril diameter in an uninjured cornea is 25-35nm, with fibres arranged in parallel to form layers (lamellae) that are 200-250nm thick. The collagen fibrils are considered weak scatterers of light as their diameters are less than that of the wavelength of light (13).
This suggests, therefore, that disruption to this arrangement is responsible at least in part for loss of transparency and therefore hazing after injury. Indeed, it has been shown that collagen, along with the rest of the ECM, are deposited in a more disorganised way following corneal wounding (16); an X-ray diffraction study conducted in 1994 shows that the collagen fibrils produced in rabbit corneas after injury show an elevation in interfibrillar spacing and an increase in fibril diameter (17).
It is thought that the increase in interfibrillar spacing could be due to the presence of stromal ‘lakes’, or spaces in the newly deposited ECM where there are no collagen fibrils present – these spaces have an increased light scattering effect and cause a cloudy appearance (18).
Proteoglycans, another component of the ECM produced by keratocytes, are responsible for regulating the diameter and spacing of collagen fibrils, and therefore can also be considered to play a role in producing haze related to collagen organisation.
One study from 1983 has suggested that in the repairing cornea, a down regulation of the keratan sulfate proteoglycan can be seen, which is normally responsible for controlling fibril diameter, accompanied by an upregulation of other proteoglycan types (19). This suggests a possible link between proteoglycan composition and corneal transparency.
Despite the suggestion from various sources that collagen fibril arrangement and the presence of lakes plays a role in light scattering and therefore opacity, more recent studies have suggested that this is not, in fact, the case.
In 2003, a study was conducted using the right eyes of four young rabbits which showed that following phototherapeutic keratectomy, although fibrils were more disorganised and interfibrillar spacing increased, there was no change in the level of transparency in these corneas (20).
This suggests that collagen organisation does not, in fact, contribute to haze. An article published in 2015 suggests that the theory that collagen fibril organisation is critical for maintaining transparency would only be acceptable if the stroma was acellular, as the presence of any cells would disturb the short-range order of the stroma (21).
Given that it is well known that the stroma in fact contains keratocytes, the theory doesn’t fit. It is suggested instead that the highly uniform organisation of the collagen fibrils is instead important for strength and regulation of corneal curvature, rather than transparency (22).
It can be concluded therefore that while much of the literature suggests that one cause of haze could be loss of the regularity of the collagenous stromal lamellae, more recent research suggests that this is not the case.
The Role of Neutrophils
Neutrophils are cells with a critical role in the inflammatory response after corneal injury, as well as an involvement in wound healing. They are the first leukocytes to reach the injured tissue and typically migrate to the corneal stroma within a few hours of injury via the limbal vessels. Their precise role in corneal healing is controversial; it is known that they release inflammatory cytokines to initiate, amplify and eventually resolve the inflammatory response, and play a role in clearing cellular debris and microbes (23).
However, there is evidence to suggest that exaggerated or prolonged neutrophil action exacerbates the inflammatory response and results in damage, due to the release of oxidative and hydrolytic enzymes that may damage otherwise healthy corneal tissue. Some studies suggest that a reduced immune response, including reduced neutrophil infiltrate, causes more rapid wound healing, for example in alkali-burned corneas of mice in a study conducted in 2005 (24). Conversely, others suggest that depletion of neutrophils slows healing of the corneal surface. For example, a study was published six years earlier investigating the effect of an absence of neutrophils in rabbit corneas, some having received photorefractive keratectomy and some alkali-burns. The neutropenia in this case was found to be associated with delayed wound healing (25).
Due to this uncertainty regarding the effect of neutrophils on wound healing in general, the matter of neutrophils’ role in corneal scarring is even more unclear. One study, published in March 2017, examined the effects of corneal stromal stem cells (CSSCs) on corneal scarring (26).
Wounded mouse corneas were treated with CSSCs which acted to inhibit neutrophil infiltration into the cornea, due to being blocked by a protein, TSG-6, secreted by the CSSCs. The results showed a significant reduction in scarring of corneas treated in this way. Prior to this study there was virtually no existing data on the role of neutrophils in corneal scarring, so it provided a novel perspective and little is known about the mechanisms that may underlie the process.
A study was conducted in 2003 with mice corneas that contained no functioning neutrophils or macrophages, and showed that wound repair could occur without the inflammatory response, and that the healed cornea was free from scarring (27). This also perhaps offers evidence to support the role of neutrophils in scarring. Beyond this, however, there is very little evidence documenting neutrophils’ role in scar formation, and a great deal more research is required to elucidate the mechanisms that may be responsible for this phenomenon, but what little existing evidence there is does appear to point to a contribution of neutrophils to the process.
After thorough examination of the relevant literature, the three predominant factors thought to lead to corneal scarring – keratocyte activation by growth factors, collagen fibril organisation and the role of neutrophils – have been identified and their respective importance assessed. The literature overwhelmingly suggests that keratocyte transformation to light-scattering repair myofibroblasts is the most important mechanism(2), and there is a great deal of authoritative evidence to support this. With this knowledge, methods of reducing scarring have been developed, such as TGFb3 stimulation(15) and TGFb-neutralizing antibodies (14). Further research into ways to inhibit this mechanism holds a great deal of potential for bringing feasible therapies to prevent scarring into clinical practice.
There is now considerable evidence suggesting that disruption to collagen fibril organisation does not in fact cause hazing. However, further research to explain why it was previously thought to be the case, and to confirm the latest view that fibril organisation plays no part in hazing, would nonetheless be useful to aid our understanding of the mechanisms leading to haze.
With regards to neutrophils’ role in producing haze after wound healing, much more research is required to produce convincing evidence of their proposed role. If sufficient research was conducted and the underlying mechanisms elucidated, inhibition of neutrophil action could also provide a target for treatment to prevent corneal haze in the future.
References
- Lee TN. The ins and outs of corneal wound healing. [internet]. 2016. [cited 2017 March 1]; [1 page]. Available from: https://www.reviewofoptometry.com/article/the-ins-and-outs-of-corneal-wound-healing
- Wilson SL, El Haj AJ, and Yang Y. Control of scar tissue formation in the cornea: strategies in clinical and cornea tissue engineering. J Funct. Biomater. [internet]. 2012 September 18 [cited 2017 March 1]; 3(3): 642-687. Available from: https://www.mdpi.com/2079-4983/3/3/642/htm#B39-jfb-03-00642
- Steele, C. Corneal wound healing: a review. Optometry Today [Internet]. 1999 September 24 [cited 2017 March 1]; 28-32. Available from: https://ot.kenthouse.com/uploads/articles/08f2dd1c8e4b1478d82662b59289398c_Steele1990924.pdf
- McLaren JW, Wacker K, Kane KM and Patel SV. Measuring corneal haze by using Scheimpflug photography and confocal microscopy. Invest Ophthalmol Vis Sci [internet]. 2016 January 22 [cited 2017 March 1]; 57(1): 227-235. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4727526/
- Texas school for the blind and visually impaired. Corneal scarring. [internet]. 2017 [cited 2017 March 1]; [1 page]. Available from: https://www.tsbvi.edu/assessment/43-archives/979-corneal-scarring
- Dua HS, Gomes JA and Singh A. Corneal epithelial wound healing. Br J Ophthalmol [internet]. 1994 May [cited 2017 March 1]; 78(5): 401-408. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC504797/?page=2
- Crosson CE, Klyce SD and Beuerman RW. Epithelial wound closure in the rabbit cornea: a biphasic process. Invest Ophthalmol Vis Sci [internet]. 1986 April [cited 2017 March 2]; 27(4): 464-473. Available from: https://www.ncbi.nlm.nih.gov/pubmed/3957565
- He J and Bazan HEP. Epidermal growth factor induces corneal keratocyte differentiation via PI-3 kinase activity. Invest Ophthalmol Vis Sci [internet]. 2009 January 6 [cited 2017 March 2]; 49(7): 2936-2945. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2614374/
- Nakamura K, Kurosaka D, Yoshino M, Oshima T and Kurosaka H. Injured corneal epithelial cells promote myodifferentiation of corneal fibroblasts. Invest Ophthalmol Vis Sci [internet]. 2002 August [cited 2017 March 2]; 43(8): 2603-2608. Available from: https://iovs.arvojournals.org/article.aspx?articleid=2123602
- Austin J. Corneal injuries and wound healing – process and therapies. Clin Opthalmol [internet]. 2014 March 21 [cited 2017 March 2]; 1(4): 1017. Available from: https://austinpublishinggroup.com/clinical-ophthalmology/fulltext/ajco-v1-id1017.php
- Zarbin M and Chu D. Mitomycin C in Corneal Refractive Surgery. Surv Opthalmol [internet]. 2009 August [cited 2017 March 2]; 54(4): 487-502. Available from: https://www.escrs.org/vienna2011/programme/handouts/IC-50/IC-50_deBenito-Llopis%26%20Teus_Handout%201.pdf
- Torricelli AAM, Singh V, Santhiago MR and Wilson SE. The corneal epithelial basement membrane: structure, function and disease. Invest Ophthalmol Vis Sci [internet]. 2013 September 27 [cited 2017 March 2]; 54(9): 6390-6400. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3787659/
- Wilson SL, El Haj AJ and Yang Y. Control of scar tissue formation in the cornea: strategies in clinical and corneal tissue engineering. J Function Biomater [internet]. 2012 September 18 [cited 2017 March 2]; 3(3): 642-687. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4031002/#B54-jfb-03-00642
- Cordeiro MF, Schultz GS, Ali RR, Bhattacharya SS and Khaw PT. Molecular therapy in ocular wound healing. J Ophthalmol [internet]. 1999 [cited 2017 March 2]; 83(1): 1219-1224. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1722849/pdf/v083p01219.pdf
- Karamichos D, Hutcheon AEK and Zieske JD. Transforming growth factor-b3 regulates assembly of a non-fibrotic matrix in a 3D corneal model. J Tissue Eng Regen Med [internet]. 2011 August [cited 2017 March 2]; 5(8): 589-672. Available from: https://www.ncbi.nlm.nih.gov/pubmed/21604386
- Saeidi N, Guo X, Hutcheon AEK, Sander EA, Bale SS, Melotti SA et al. Disorganised collagen scaffold interferes with fibroblast mediated deposition of organised extracellular matrix in vitro. Biotechnol Bioeng [internet]. 2012 May 4 [cited 2017 March 2]; 109(10): 2683-2698. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3757098/
- Rawe IM, Meek KM, Leonard DW, Takahashi T and Charles C. Structure of corneal scar tissue: an X-ray diffraction study. Biophysical Journal [internet]. 1994 October [cited 2017 March 2]; 67(1): 1743-1748. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1225536/pdf/biophysj00070-0381.pdf
- Rawe IM, Zabel RW, Tuft SJ, Chen V and Meek KM. A morphological study of rabbit corneas after laser keratectomy. Eye [internet]. 1992 [cited 2017 March 2]; 6(1): 637-642. Available from: https://www.nature.com/eye/journal/v6/n6/full/eye1992137a.html
- Hassell JR, Cintron C, Kublin C and Newsome DA. Proteoglycan changes during restoration of transparency in corneal scars. Arch Biochem Biophys [internet]. 1983 April 15 [cited 2017 March 2]; 222(2): 362-369. Available from: https://ac.els-cdn.com/0003986183905325/1-s2.0-0003986183905325-main.pdf?_tid=fa9cfaf2-041a-11e7-aae9-00000aacb362&acdnat=1488990138_8de8a7d6dbc5267d4e47f18c0b202b62
- Connon CJ, Marshall J, Patmore AL and Meek KM. Persistent haze and disorganisation of anterior stromal collagen appear unrelated following phototherapeutic keratectomy. J Refract Surg [internet]. 2003 May [cited 2017 March 2]; 19(3): 323-332. Available from: https://www.healio.com/ophthalmology/journals/jrs/2003-5-19-3/%7B23331240-bd18-4c58-bd2d-f5e6a9904c89%7D/persistent-haze-and-disorganization-of-anterior-stromal-collagen-appear-unrelated-following-phototherapeutic-keratectomy
- Gardner S. Studies of corneal structure and transparency. [PhD thesis] [internet]. Cardiff (UK): Cardiff University; 2015. Available from: https://orca.cf.ac.uk/73643/
- Chen S, Mienaltowskik MJ and Birk DE. Regulation of corneal stroma extracellular matrix assembly. Exp Eye Res [internet]. 2015 April [cited 2017 March 3]; 133(1): 69-80. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25819456
- Marrazzo G, Bellner L, Halilovic A, Volti GL, Drago F, Dunn MW et al. The role of neutrophils in corneal wound healing in HO-2 null mice. PLoS One [internet]. 2011 June 17 [cited 2017 March 3]; 6(6): 1371-1412. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0021180
- Ueno M, yons BL, Burzenski LM, Gott B, Shaffer DJ, Roopenian DC and Schultz LD. Accelerated wound healing of alkali-burned corneas in MRL mice is associated with a reduced inflammatory signature. Invest Opthalmol Vis Sci [internet]. 2005 November [cited 2017 March 3]; 46(11): 4097-4106. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16249486
- Gan L, Fagerholm P and Kim HJ. Effect of leukocytes on corneal cellular proliferation and wound healing. Invest Ophthalmol Vis Sci [internet]. 1999 March [cited 2017 March 3]; 40(3): 575-581. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10067960
- Hertsenberg AJ, Shojaati G, Funderburgh ML, Mann MM, Du Y and Funderburgh JL. Corneal stromal stem cells reduce corneal scarring by mediating neutrophil infiltration after wounding.PLoS One [internet]. 2017 March 3 [cited 2017 March 4]; 12(3): e0171712. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28257425
- Martin P, D’Souza D, Martin J, Grose R, Cooper L, Maki R et al. Wound healing in the PU.1 null mouse- tissue repair is not dependent on inflammatory cells. Curr Biol [internet]. 2003 July 1 [cited 2017 March 4]; 13(13): 1122-1128. Available from: https://www.ncbi.nlm.nih.gov/pubmed/12842011
