Freddie Bailey1, Edward Pritchard1, Michael O’Gallagher1
Affiliations:
1Royal Victoria Hospital Belfast, 274 Grosvenor Road, Belfast, BT12 6BA
Corresponding Author:
Mr Michael O’Gallagher, Consultant Ophthalmologist ([email protected])
Keratoconus
Keratoconus is a bilateral asymmetric chronic disease process characterised by progressive corneal thinning that results in irregular astigmatism and decreased visual acuity (1). The pathophysiology of keratoconus begins with a reduction in collagen lamellae within Bowman’s membrane (the second outermost of the five corneal layers, between the outer corneal epithelium and middle stromal layers) (1). Progression of disease then affects the deepest corneal endothelial layer, distorting the morphology and tessellation of normal hexagonal shaped corneal endothelial cells (reduced pleomorphism), increasing the variation in cell size (polymegathism), but without affecting the overall number of corneal endothelial cells (2). In the UK, keratoconus has been shown to have a marked ethnicity split, with incidence in Caucasian British populations (3.3-4.5/100,000 population/year) significantly lower than in British Asian populations (19.6-25/100,000 population/year) (3). Its onset and progression is usually in the second and third decades of life, with subsequent stabilisation thereafter, although progression may also occur in older affected individuals (4). Keratoconus management is usually conservative through monitoring and refractive correction, with complications such as corneal hydrops dealt with separately, and surgical treatment such as corneal collagen cross-linking, deep anterior lamellar keratoplasty (DALK), and penetrating keratoplasty (PK) usually reserved for those with more severe disease.
Case report
A 42 year old Caucasian male was referred to the cornea clinic after his optician noticed early signs of keratoconus – Increased irregular astigmatism. The patient had no significant past medical or ocular history. Interestingly, the patient was a carrier of the mitochondrial DNA mutation m.3243A>G. None of his affected relatives had clinical ocular involvement.
The patient was asymptomatic. His visual acuity was intact (6/6 unaided right eye; 6/4 unaided left eye). His prescription showed mild myopic astigmatism (right eye -0.75/-1.50/45°; left eye -0.75/-0.75/125°). He was found to have split reflexes bilaterally on retinoscopy in keeping with keratoconus. He had mild meibomian gland dysfunction, and a small area of granulation tissue on his right upper eyelid. There were a few subtle Vogt’s striae on his right cornea (fine vertical parallel lines caused by undulations of collagen lamellae in the corneal stroma) – A clinical sign also in keeping with keratoconus.
Pentacam demonstrated bilateral corneal thinning (thinnest location right cornea 462 µm; left cornea 453 µm; normal ≥ 470 µm) and irregular topographic astigmatism (right cornea 2.8 D at 32.9°; left cornea 2.0 D at 156.3°; normal ≤ 1.0 D astigmatism and ≤ 15° axis) (5). A diagnosis of keratoconus was therefore made. However, as his inferior cones left the central visual axis relatively spherically, this explained his lack of visual symptoms (see Figure 1).
Interestingly, the patient was also found to have a relatively reduced corneal endothelial cell count for his age (2032 cells/mm2 right; 2070 cells/mm2 left), but with a normal degree of polymegathism (right cornea 29%; left cornea 28%; normal ≤ 32% for ages 40-49) and pleomorphism (right cornea 60%; left cornea 69%; normal ≥ 60% for ages 40-49) (6). This is an interesting finding as the corneal endothelium is non-regenerative, with the highest endothelial cell counts being found in childhood, and reductions seen throughout life (2931 cells/mm2 ± 371 for ages 20-29; 2660 cells/mm2 ± 301 for ages 40-49; 2222 cells/mm2 ± 182 for ages 80-89) (6). Corneal endothelial cell counts are further reduced and disordered by trauma, inflammation, and a range of primary and secondary corneal endothelial disorders (6). Overall, this suggests that while reduced, the corneal endothelial cells were not overtly pathological (see Figure 2).
The patient was informed that, given his age, he was unlikely to suffer any progression of disease. Nevertheless, a repeat Pentacam scan in 1 year was arranged to monitor his progress given the possible links between his mitochondrial mutation and his corneal changes.
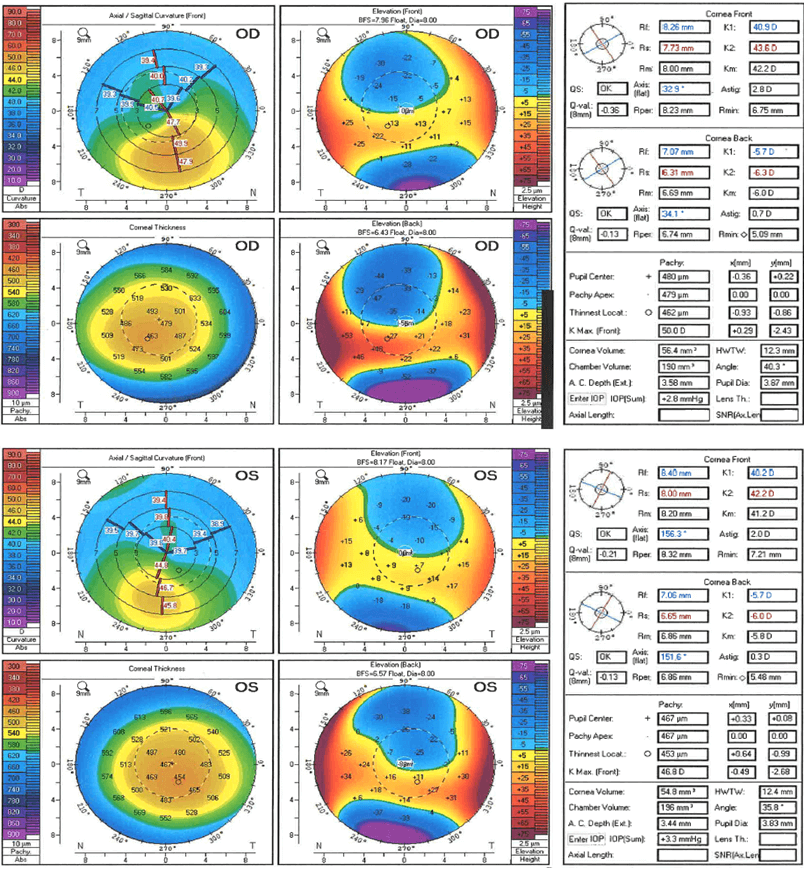
Figure 1. Pentacam image demonstrating abnormal corneal thinning (thinnest location right 462 µm; left 453 µm) and irregular topographic astigmatism (right 2.8 D at 32.9°; left 2.0 D at 156.3°) in a patient with mitochondrial DNA mutation m.3243A>G.
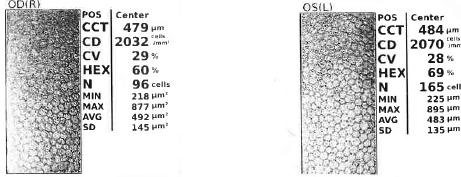
Figure 2. Specular microscopy of corneal endothelium demonstrating relatively low corneal endothelial cell counts in a patient with mitochondrial DNA mutation m.3243A>G.
The role of mitochondrial DNA mutation m.3243A>G
Previous genetic studies of keratoconus have focused on the human genome to limited success (7). Mitochondria produce over 90% of total cellular energy in the form of adenosine triphosphate (ATP). Mitochondrial DNA mutations therefore affect tissues with the highest metabolic activity. Within the eye, the retina and optic nerve (8), as well as the corneal endothelium (9), are tissues with the highest metabolic activity. One of the commonest point mutations of the mitochondrial DNA, m.3242A>G, has a variable maternal inheritance (mitochondrial DNA is only found in the ovum, not the sperm) (10). The m.3242A>G mutation is associated in 30% of cases of “MIDD” (Maternally Inherited Diabetes and Deafness) and in 10% of the “MELAS” (Mitochondrial Encephalopathy with Lactic Acidosis and Stroke-like episodes) syndromes (11). However, it is thought that the overall population carrier rate of this mutation is 1 in 400 people in the UK (12). This suggests that a large number of carriers do not go on to develop a syndromic disease.
Previously described syndromic and non-syndromic ocular manifestations of the m.3243A>G mutation have expectedly been limited to areas of high metabolic activity – The retina, the optic nerve, the extra-ocular muscles (8), and the corneal endothelium (13,14). However, to our knowledge, there have been no cases of keratoconus associated with this relatively common mitochondrial mutation. In a post-mortem study of patients with MELAS syndrome, abnormal mitochondria were found throughout all layers of the cornea (13). In a study of patients aged 41-60 with non-syndromic m.3243A>G mutations, the corneal endothelial cell count and degree of pleomorphism were normal, but increased polymegathism was present (14). This contrasts with the findings in this case report of reduced endothelial cell counts but normal degrees of polymegathism and pleomorphism, and suggests a possible association with the m.3243A>G mutation and variable subclinical corneal endothelial dysfunction.
Conclusion
To our knowledge, this is the first case report of a possible association of subclinical keratoconus and mitochondrial DNA mutation m.3243A>G. This case underlines the importance of taking a detailed family history from patients presenting to ophthalmology clinic. It also emphasises the importance of elucidating possible mitochondrial genetic links to ophthalmological conditions such as keratoconus where human genetic links have yet to be proven.
References
- Davidson AE, Hayes S, Hardcastle AJ, Tuft SJ. The pathogenesis of keratoconus1. Eye (Lond). 2014 Feb;28(2):189-95. doi: 10.1038/eye.2013.2782. Epub 2013 Dec 20. PMID: 24357835.
- Elmassry A, Osman A, Sabry M, Elmassry M, Katkat M, Hatata MY, El-Kateb M. Corneal endothelial cells changes in different stages of Keratoconus: a multi-Centre clinical study. BMC Ophthalmol. 2021 Mar 21;21(1):143. doi: 10.1186/s12886-021-01913-73. PMID: 33743631.
- Santodomingo-Rubido J, Carracedo G, Suzaki A, Villa-Collar C, Vincent SJ, Wolffsohn JS. Keratoconus: An updated review4. Cont Lens Anterior Eye. 2022 Jun;45(3):101559. doi: 10.1016/j.clae.2021.1015595. Epub 2022 Jan 4. PMID: 34991971.
- Gokul A, Patel DV, Watters GA, McGhee CNJ. The natural history of corneal topographic progression of keratoconus after age 30 years in non-contact lens wearers6. Br J Ophthalmol. 2017 Jun;101(6):839-844. doi: 10.1136/bjophthalmol-2016-3086827. Epub 2016 Oct 11. PMID: 27729309.
- Fung, T.H.M., Amoaku, W.M.K. (2020)8. Patient Investigations and Interpretation9. In: Fung, T., Amoaku, W. (eds) Viva and OSCE Exams in Ophthalmology10. Springer, Cham. https://doi.org/10.1007/978-3-030-43063-4_2
- Galgauskas S, Norvydaitė D, Krasauskaitė D, Stech S, Ašoklis RS. Age-related changes in corneal thickness and endothelial characteristics11. Clin Interv Aging. 2013;8:1445-50. doi: 10.2147/CIA.S5169312. Epub 2013 Oct 24. PMID: 24187493.
- Lucas SEM, Burdon KP. Genetic and Environmental Risk Factors for Keratoconus13. Annu Rev Vis Sci. 2020 Sep 15;6:25-46. doi: 10.1146/annurev-vision-121219-08172314. Epub 2020 Apr 22. PMID: 32320633.
- Chen BS, Harvey JP, Gilhooley MJ, Jurkute N, Yu-Wai-Man P. Mitochondria and the eye-manifestations of mitochondrial diseases and their management15. Eye (Lond). 2023 Aug;37(12):2416-2425. doi: 10.1038/s41433-023-02523-x16. Epub 2023 Apr 2517. PMID: 37185957.
- Vallabh NA, Romano V, Willoughby CE. Mitochondrial dysfunction and oxidative stress in corneal disease18. Mitochondrion. 2017 Sep;36:103-113. doi: 10.1016/j.mito.2017.05.00919. Epub 2017 May 23. PMID: 28549842.
- de Laat P, Koene S, Heuvel LP, Rodenburg RJ, Janssen MC, Smeitink JA. Inheritance of the m.3243A>G mutation20. JIMD Rep. 2013;8:47-50. doi: 10.1007/8904_2012_15921. Epub 2012 Jul 6. PMID: 23430519.
- Nesbitt V, Pitceathly RD, Turnbull DM, Taylor RW, Sweeney MG, Mudanohwo EE, Rahman S, Hanna MG, McFarland R22. The UK MRC Mitochondrial Disease Patient Cohort Study: clinical phenotypes associated with the m.3243A>G mutation–implications for diagnosis and management23. J Neurol Neurosurg Psychiatry. 2013 Aug;84(8):936-8. doi: 10.1136/jnnp-2012-30352824. Epub 2013 Jan 25. PMID: 23355809.
- Elliott HR, Samuels DC, Eden JA, Relton CL, Chinnery PF25. Pathogenic mitochondrial DNA mutations are common in the general population26. Am J Hum Genet. 2008 Aug;83(2):254-60. doi: 10.1016/j.ajhg.2008.07.00427. PMID: 18674747.
- Rummelt V, Folberg R, Ionasescu V, Yi H, Moore KC. Ocular pathology of MELAS syndrome with mitochondrial DNA nucleotide 3243 point mutation28. Ophthalmology. 1993 Dec;100(12):1757-66. doi: 10.1016/s0161-6420(13)31404-329. PMID: 8259272.
- Bakhoum MF, Wu WP, White EC, Sengillo JD, Sanfilippo C, Morcos MM, Freund KB, Perry HD, Sarraf D, Tsang SH30. Mitochondrial A3243G mutation results in corneal endothelial polymegathism31. Graefes Arch Clin Exp Ophthalmol. 2018 Mar;256(3):583-588. doi: 10.1007/s00417-018-3914-z32. Epub 2018 Jan 29. PMID: 29376197.
