Madiah Mahmood
A minimally invasive cosmetic treatment also known as a non-surgical procedure (NSP) refers to a procedure which involves small incisions or skin punctures intended to produce an aesthetic change with ‘minimal risk and downtime’ (1). Dermal filler is used in multiple NSP, for example in the glabellar region to “treat” deep-frown lines, in non-surgical rhinoplasty, in the tear-trough region to reduce hollowness of the tear trough and reduce under-eye circles and in the forehead for brow lifts (2,3). NSP in these areas have associated complications related to the eye including blindness and optic neuropathy (4,5,6).
Currently medical professional and non-medically trained professionals can provide NSP for aesthetic purposes which means some practitioners may lack knowledge of facial anatomy and how to respond if complications occur. The British Association of Aesthetic Plastic Surgeons found that 69% of their surgeons had consulted patients presenting with complications of temporary filler (7). In 2022, Safe Face charity found 69% of the 2824 total complaints were related to dermal filler (7).
Dermal filler can comprise of hyaluronic acid, calcium hydroxyapatite, polylactic acid, silicone and polymethylmethacrylate (8). During a NSP using dermal filler, impairment, or loss of vision secondary to central retinal or retinal branch artery occlusion can occur (6). Periocular complications associated with blindness following dermal filler injections include four types (6):
- Blindness without ophthalmoplegia and ptosis
- Blindness with ptosis, without ophthalmoplegia
- Blindness with ophthalmoplegia, without ptosis
- Blindness with ophthalmoplegia and ptosis
Although previous case reports have not shown improvement of visual acuity in patients with vascular occlusion, there was dramatic recovery in ptosis and ophthalmoplegia symptoms (6).
How does the filler lead to blindness?
Blindness as a complication of dermal filler injection has been reported the most when used in the nose and glabellar region, however moderate risk regions include nasolabial folds, forehead, periocular, temple and cheek (6,9).
The ophthalmic artery has numerous branches supplying the orbital region (intra and extraocular structures) and ocular region (globe), it first branches into the central retinal artery. The variable vascular anatomy between patients means the risks associated with filler from facial vasculature to orbital vasculature can make it challenging to identify “safe regions” for injection (6,7,8). The supraorbital and supratrochlear terminal branches of the ophthalmic artery supply the forehead and anastomose with branches of the angular artery (6). (See figure 1)
Injection of dermal filler into one of these vessels may result in retrograde flow, when pressure from the syringe plunger is released, systolic pressure causes the product to flow anterograde into the ophthalmic artery or central retinal artery causing occlusion and vision loss (6).
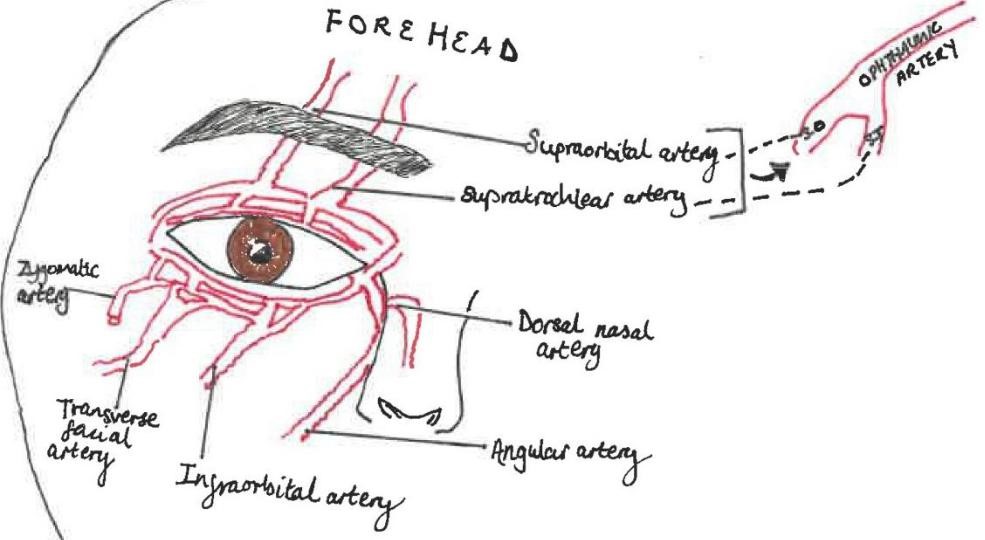
Recognising vision loss by filler
Patients who undergo NSP involving filler which is then complicated by blindness may experience vision loss, paralysis/weakness of ocular muscles, ptosis, corneal oedema and pain. Complete vision loss is the usual presentation, patients may also present with sudden onset of severe ocular or facial pain, however central retinal and retinal branch artery occlusions may present without ocular pain (6).
Initial treatment
There is a narrow timeframe of 60-90 minutes in which to treat blindness due to occlusion of the retinal artery as thereafter, blindness is irreversible (6). There are 3 things an aesthetics practitioner can do if they suspect blindness after facial fillers (6):
- Stop treatment
- Place patient in supine position
- Call 999, ask for patient to be transferred to a hospital setting as soon as possible
The aim of treatment is to reduce the intraocular pressure so the emboli can flow downstream and improve retinal perfusion; referral to a speciality eye hospital is paramount (6). Methods to improve outcomes include administering timolol drops to try and reduce intraocular pressure by reducing aqueous humour production, massaging the globe with repeated increasing pressure may help to dislodge the emboli and administering hyaluronidase if hyaluronic acid has been used although there is debate on whether this can improve vision loss (6).
Specialist treatment
Specialist treatment involves reducing intraocular pressure, removing, or reversing central retinal ischaemia and increasing blood flow to the retina (6). Table 1 demonstrates specialist treatment and its aims (6).
| Treatment | Aim |
| 500mg IV acetazolamide | Increase retinal blood flow Reduce intraocular pressure |
| Enoxaparin SC | Anticoagulation (If signs/symptoms of cerebral infarction – postpone until neuro review complete) |
| IV mannitol | Reduce intraocular pressure |
| Injection of hyaluronidase via transorbital approach into ophthalmic artery | Dissolve hyaluronic acid dermal filler |
NSP’s are on the rise, not only is it a lucrative industry currently worth around £3.6 billion whereby individuals utilise it to monopolise, but the prevalence is also increasing along with the increased influence from social media, accessibility and affordability compared to surgical procedures (10). Therefore, it is paramount practitioners have the appropriate licenses and training to deal with complications. Doctors within specialist and non-specialist ophthalmology hospitals need to have up to date guidelines, training, and equipment to prevent devastating life changing complications such as blindness as the aesthetic filler trend grows.
References
- Devgan L, Singh P, Durairaj K. 2019. Minimally invasive facial cosmetic procedures. Otolaryngologic Clinics of North America. [Online]. Volume 52 (3), 443-459. [DOA 19.10.24]Available from https://doi.org/10.1016/j.otc.2019.02.013
- Clark W N, Pan R D, Barrett M D. 2023. Facial fillers: Relevant anatomy, injection techniques and complications. World Journal of Otorhinolaryngology – head and neck surgery. [Online]. Volume 9 (3), 227-235. [DOA 19.10.24]. Available from: https://doi.org/10.1002/wjo2.126
- Kumar V, Jain A, Atre S, Shome D, Kapoor R, Doshi K, Vadera S. 2021. Non-surgical rhinoplasty using hyaluronic acid dermal fillers: A systematic review. J Cosmet Dermatol. Volume 20 (8), 2414-2424. [DOA 19.10.24]. https://doi.org/10.1111/jocd.14173
- Lim S C, Malhotra R. 2023. Delayed optic neuropathy caused by orbital hyaluronic filler injection and recovery following hyaluronidase treatment. Ophthalmic Plastic and Reconstructive Surgery. [Online]. Volume 39 (5), 155-158. [DOA 19.10.24]. Available from https://doi.org/10.1097/iop.0000000000002409
- Hu Y, Wang Y, Tong Y. 2019. Optic perineuritis secondary to hyaluronic acid injections: a case report. BMC Ophthalmology. [Online]. Volume 19 (1), 241. [DOA 19.10.24]. Available from https://doi.org/10.1186/s12886-019-1247-2
- Walker L, King M. 2018. This month’s guideline: Visual loss secondary to cosmetic filler injection. Journal of clinical and aesthetic dermatology. [Online]. Volume 11 (5) 53-55. [DOA 19.10.24]. Available from This month’s guideline: Visual Loss Secondary to Cosmetic Filler Injection – PMC (nih.gov)
- Hamilton Fraser. 2023. The Health & Care Act 2022 – Hamilton Fraser. [Online]. Available from: The Health & Care Act 2022 – Hamilton Fraser
- Tran Q A, Lee W. 2021. Vision Loss and Blindness Following Fillers. Journal of Dermatology and Skin Science. [Online]. [DOA 19/10/24]. Available fromhttps://doi.org/10.29245/2767-5092/2021/1.1134
- Zhao F, Chen Y, He D, You X, Xu Y. 2024. Disastrous cerebral and ocular vascular complications after cosmetic facial filler injections: a retrospective case series study. Scientific Reports. [Online]. Volume 14 (3495). [DOA 19/10/24]. Available from https://doi.org/10.1038/s41598-024-54202-w
- Department of Health & Social Care. 2023. The licensing of non-surgical cosmetic procedures in England. [Online]. [DOA 19.10.24] Available from The licensing of non-surgical cosmetic procedures in England – GOV.UK (www.gov.uk)
