Anastasia Margariti1 , Victoria Nowak1 , Fion Bremner1, Zhaleh Khaleeli1, Axel Petzold1
1. Ophthalmology, National Hospital for Neurology and Neurosurgery, Queen Square, LONDON, GBR
Abstract
Ocular sarcoidosis typically presents either to the uveitis or neuro-ophthalmic service, depending on the compartment affected. Here we present a case of a 70-year-old woman who developed profound right eye visual loss to hand movements over two weeks with unilateral disc swelling and optic neuropathy. OCT demonstrated the dynamics of vertically migrating intraretinal hyperreflective foci, subretinal fluid, choroidal thickening and vitreous haze. Intracranial and orbital imaging, blood tests, lumbar puncture and whole-body PET MRI excluded neoplastic, infiltrative, inflammatory, infectious and known auto-immune causes. Treatment with corticosteroids led to rapid improvement of symptoms and the final visual outcome was good: pinhole acuity 0.3 with 10/17 Ishihara plates correctly identified, though a central scotoma remained. A literature review suggests that intraretinal sarcoidosis may be a novel, OCT-supported manifestation of this systemic disease. OCT permits dynamic monitoring of the inflammatory response on a cellular level between three adjacent compartments. These findings highlight the importance of multidisciplinary management of sarcoidosis, with OCT as a central part of the standard work-up.
Introduction
Sarcoidosis is a disease of unknown aetiology with both pulmonary and extra-pulmonary manifestations (1). Reports on visual loss in sarcoidosis first appeared shortly after Schaumann recognised the systemic nature of the disease (2). Diagnostic criteria have been proposed for ocular (3) and neuro-ophthalmic (4) sarcoidosis but separating these related entities may be unhelpful. Loss of vision can result from posterior uveitis or granuloma formation in the retina, orbit or elsewhere in the visual pathway (3). Other features of ocular sarcoidosis include cystoid macular oedema, segmental periphlebitis, sometimes with peripheral “candle wax dripping”, and vitreous opacities (5). Retinal sarcoidosis can mimic symptoms of an autoimmune retinopathy such as cancer associated retinopathy (6). The gold standard for diagnosis is a tissue biopsy but this is not possible or ethical in approximately two thirds of neuro-ophthalmic cases due to the site of involvement (7). The present case illustrates novel OCT observations which can contribute to making the diagnosis and monitoring the treatment response.
Case Presentation
This 70-year-old, Austronesian woman, presented with painful, blurred vision in the right eye, with pain worsening on eye movements. She had noticed reduced visual acuity (VA) over the previous 10 days. She was seen by her optician who found right optic disc swelling and referred her to the ophthalmology team. She complained of mild dizziness and headaches associated with blurred vision but did not have nausea or vomiting; her headaches were constant, of gradual onset, and 10/10 severity. She had good appetite, no recent weight loss and no drenching night sweats, but had noticed some mild hair loss. She reported no scalp tenderness, fever or jaw claudication. She had previously been reviewed for intraocular pressure monitoring but the range had been normal (14-20 mm Hg) and she had not required medication for this.
Past medical history was notable for appendicectomy in 2017. Histology showed a low-grade mucinous neoplasm. There was also known osteoarthritis.
On examination, her high-contrast Snellen visual acuity (VA) was counting fingers (CF) in the right eye and 6/9 in the left eye. Ishihara was 0/13 in the right and 10/13 in the left.
Confrontation visual fields showed severe constriction in the right eye but no defect on the left. Hertel exophthalmometry (base 113) was right 14 mm and left 13 mm. She had full, but painful eye movements.
On retropulsion, fullness of the right orbit was noted with no associated tenderness. Both superficial temporal arteries were soft to palpation, painless and pulsatile.
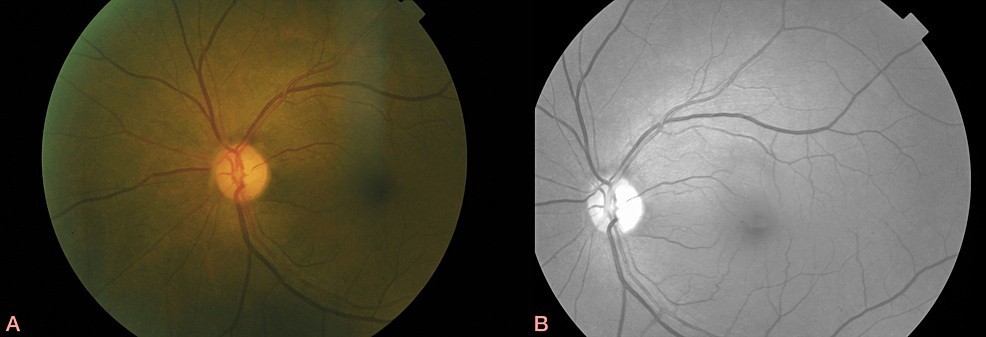
The slit lamp examination revealed right eye pseudoexfoliation and bilateral early cataracts. The intraocular pressure was 14 mm Hg on the right and 15 mm Hg on the left. The dilated fundoscopy did not reveal any inflammation in the anterior chamber or vitreous but confirmed right optic disc swelling with a few peripapillary haemorrhages and some intra- and sub-retinal fluid extending to the macula with perifoveal hard exudates (Figure 1). The peripheral retina was flat. In her left eye, disc and macula examination was normal (Figure 2), though she had a few drusen in her mid-periphery.
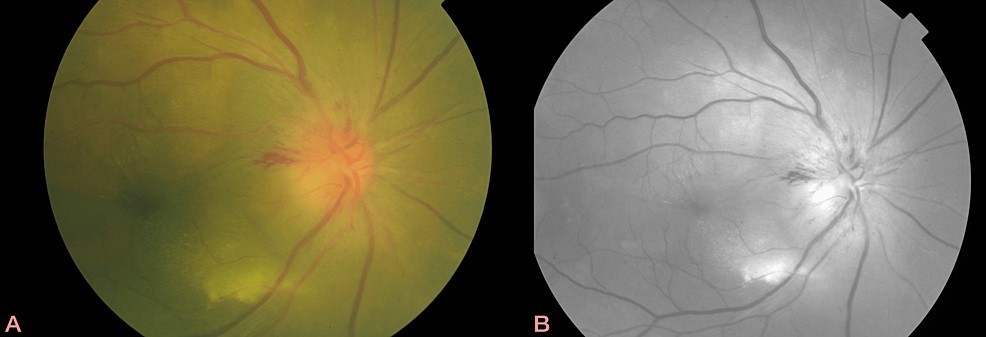
FIGURE 1: Colour (A) fundus and infrared (B) photographs of right eye showing multifocal, yellow lesions and optic disc haemorrhages at 1 and 9 o’clock.
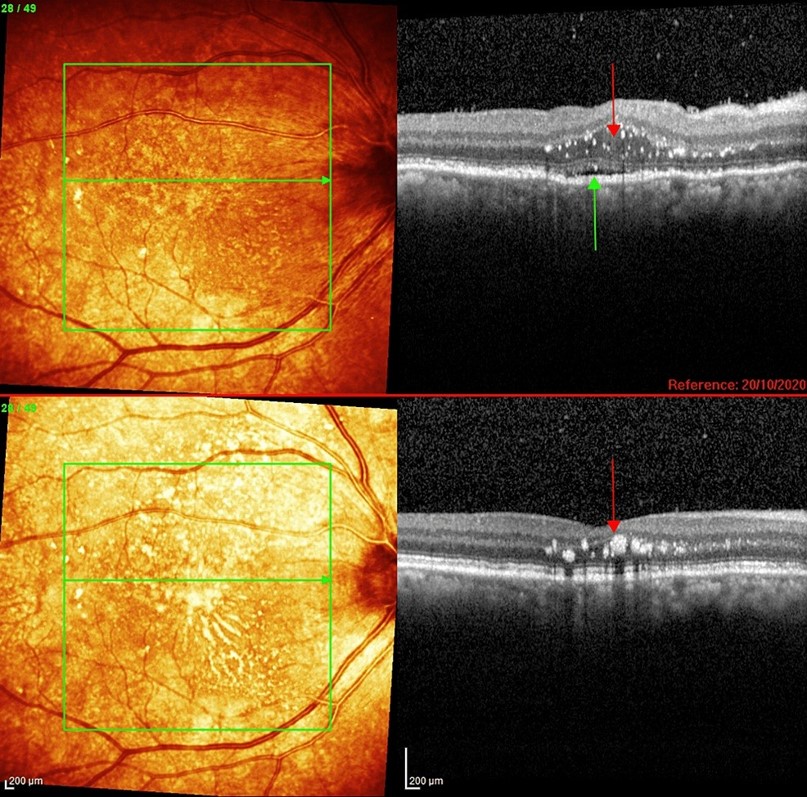
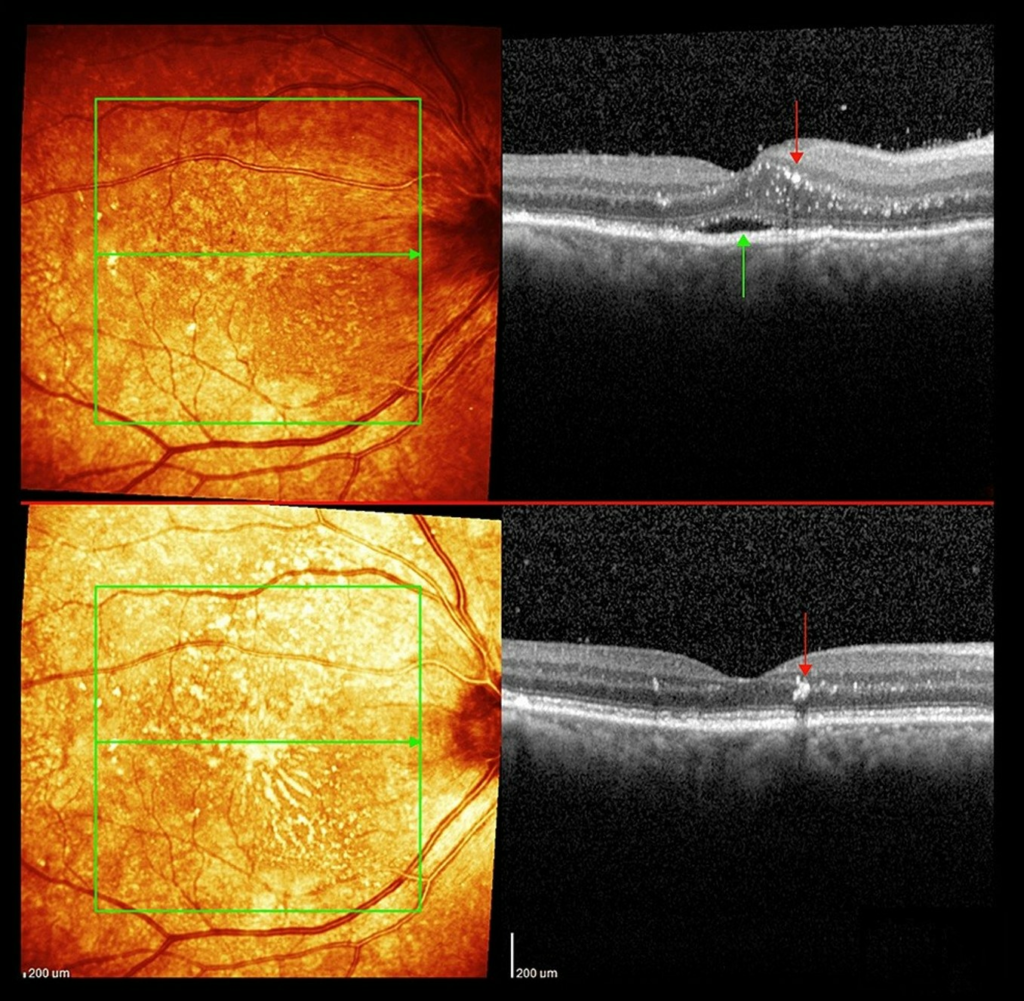
OCT showed a diffusely thickened choroid on the right and subretinal fluid without loss of retinal architecture. Swelling of the outer and inner nuclear layers close to the optic disc was also noted (Figure 3). Choroidal folds were-visible at the slit lamp and clearly seen on the OCT with RPE undulations (Figure 4). OCT also revealed vertically migrating hyperreflective foci throughout the outer nuclear layer, extending to and including the outer plexiform layer (Figure 5). The left OCT was normal (Figure 6).
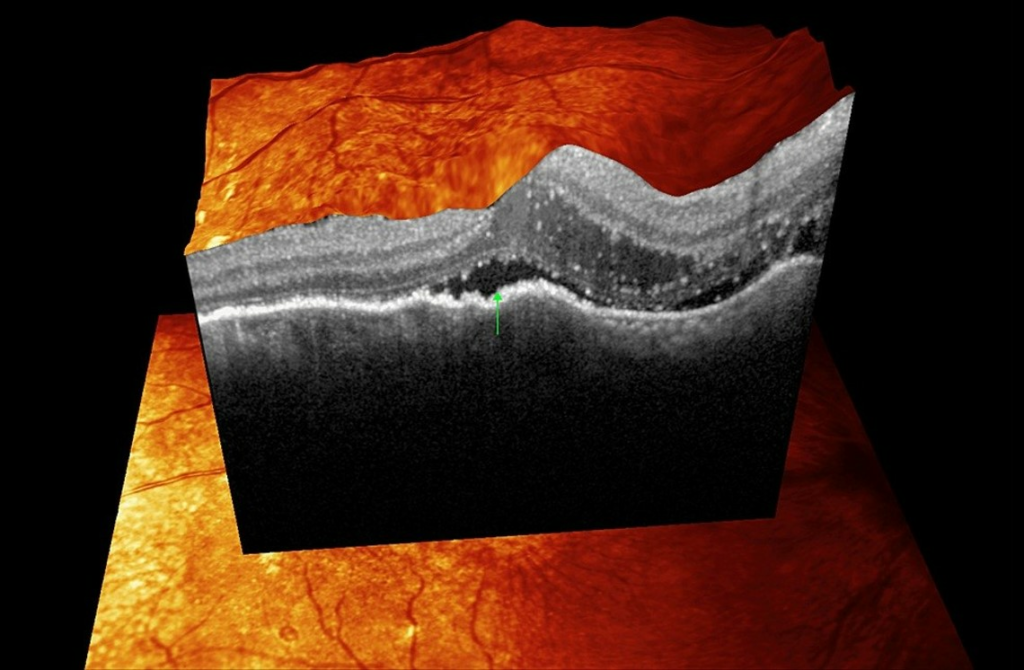
FIGURE 3: Optical coherence tomography of the right eye macula showing hyperreflective spots mainly within the outer nuclear layer. There is also subretinal fluid (green arrows) in the foveola and swelling of the outer and inner nuclear layers close to the optic disc.
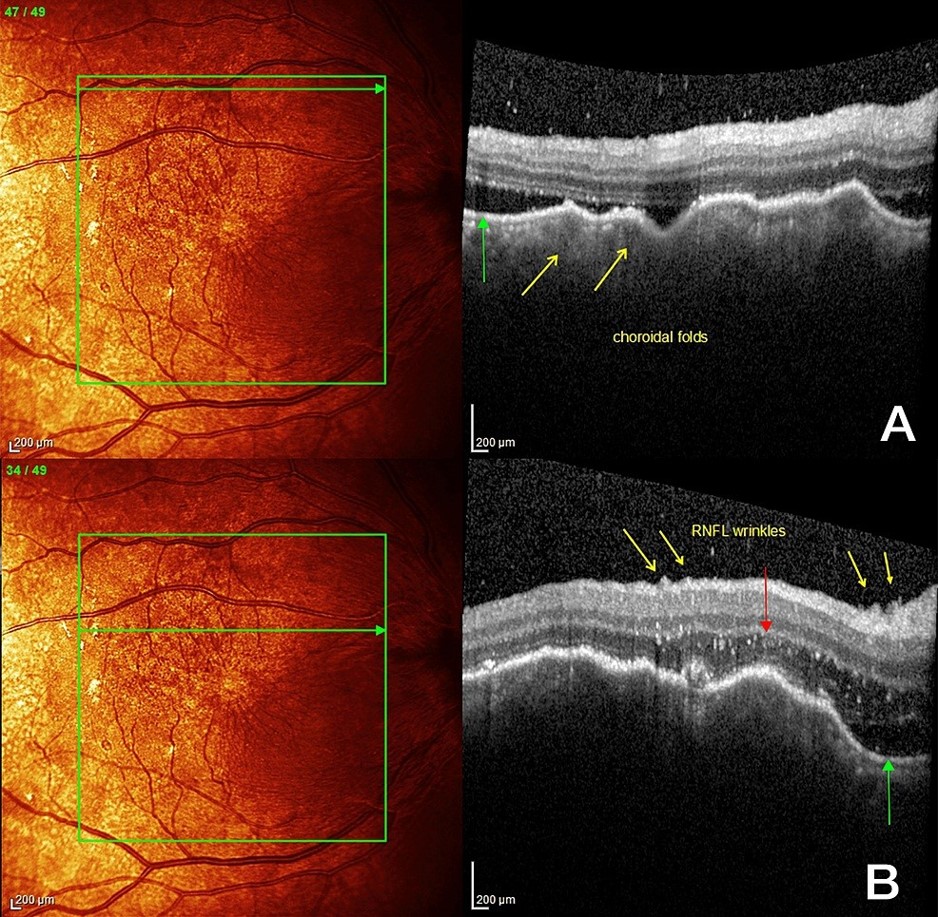
FIGURE 4: Right macula at the start of corticosteroid treatment showing: (A): Choroidal folds (yellow arrows) with subretinal fluid (green arrow) and vitritis (white spots in vitreous, compare to left eye for normal vitreous appearance), (B) Retinal nerve fibre layer wrinkles (yellow arrows) with more choroidal folds, hyper-reflective spots (red arrow)swelling of the outer nuclear layer and subretinal fluid(green arrow).
The B-scan ultrasonography confirmed a thickened right choroid with fairly homogenous echogenicity.
The results of her blood tests, including screening for paraneoplastic antibodies, were normal, except for a mildly elevated ACE and granulocytosis with eosinophilia (see Table 1).
| Blood test | Result (normal range) |
| ESR | 24 mm/h |
| CRP | 4.6 |
| WCC eosinophils | *10.31 x10^9/L (ULN 10) *- 0.45×10^9/L (ULN 0.4) |
| Other components of FBC | Normal |
| ANA | Negative |
| ENA | Negative |
| ANCA | Negative |
| Anti-JO-1 Ab | Negative |
| Anti-LA Ab | Negative |
| Anti-RNP Ab | Negative |
| Anti-RO Ab | Negative |
| Anti-Scl-70 Ab | Negative |
| Anti-SM Ab | Negative |
| Centromere antibody | Negative |
| ACE | *57.1 (normal range 8-52 U/L) |
| VDRL | Negative |
| HIV | Negative |
| Anti-AQ4-Ab | Negative |
| Anti-MOG-Ab | Negative |
| Corrected calcium | 2.56 (2.20-2.60 mmol/L) |
| CSF sample | |
| CSF IgG (Serum IgG) | 35 (13.0) |
| CSF Albumin (Serum albumin) | 270 (43) |
| CSF oligoclonal bands (Serum oligoclonal bands) | Negative (negative) |
| Neurofilament | *3548 pg/mL |
| Gram stain, microscopy and culture | No organisms, no growth |
| Protein | 0.4 g/L |
| RBC | <2/mm |
| WCC | <1/mm |
| Viral screen (CMV, EBV, HSV, VZV, Enterovirus, Parechovirus) | Not detected |
Abbreviations: ULN = upper limit of normal, Ab=Antibody, ESR=Erythrocyte Sedimentation Rate, CRP=C-reactive protein , FBC=Full blood count, WCC=white cell count, RBC=red blood cells, ANA= Antinuclear Antibody, ENA=extractable nuclear antigen, ANCA=Antineutrophilic cytoplasmic antibody, MOG=Myelin Oligodendrocyte Glycoprotein, AQ4= Aquoporin-4, Jo-1=histidyl tRNA synthetase, RNP=Ribonucleoprotein, HIV=human immunodeficiency virus, VDRL=Venereal Disease Research Laboratory, CMV=cytomegalovirus, EBV=Epstein-Barr virus, HSV=herpes simplex virus, VZV=Varicella Zoster Virus, ACE=angiotensin-converting enzyme, CSF=Cerebrospinal fluid
* denotes a value outside the normal range.
There was a broad differential diagnosis (8), (see Table 2), including paraneoplastic phenomenon; which was initially considered top of the list.
| Condition | Comments |
|---|---|
| Papilloedema | Idiopathic intracranial hypertension, Hydrocephalus |
| Infection | Tuberculosis, Syphilis, Bartonella, Lyme, Toxoplasmosis |
| Infiltration | Neoplasia, Sarcoidosis, Lymphoma |
| Inflammation | Multiple sclerosis, Neuromyelitis optica spectrum disorder, Neuromyelitis optica, Pachymeningitis, Vasculitis, Orbital pseudotumor, Diabetic papillopathy, Vogt-Koyanagi-Harada, Posterior scleritis, Thyroid-related |
| Autoimmune | Vasculitis |
| Vascular | Cerebral venous sinus thrombosis, Arteritic ischaemic optic neuropathy, Non-arteritic anterior ischaemic optic neuropathy, Central retinal vein occlusion, Malignant hypertension |
| Compressive optic neuropathy | Orbit apex syndrome |
Several investigations were required to exclude potentially life-threatening aetiologies. She was urgently admitted for further investigation. Brain imaging was performed, with CT and subsequent gadolinium- enhanced MR head/orbit/MRV/MRA. Imaging did not reveal any relevant pathology. Specifically, there were no intracranial or orbital space occupying lesions or infiltration; there was no optic pathway thickening/ enhancement, extraocular muscle enlargement, enhancement of other cranial nerves or cavernous sinus, lacrimal gland enlargement or enhancement, periventricular white matter lesions, leptomeningeal or pachymeningeal enhancement (9). There was an incidental, small suprasellar lesion of uncertain clinical significance and mild small vessel disease.
Over the following two days the visual acuity on the right worsened to hand movements. A lumbar puncture was performed. The routine cerebrospinal fluid (CSF) was normal. Extended CSF examinations included a non-disease-specific biomarker for neurodegeneration, neurofilaments (Nf). Interpretation of elevated Nf concentrations need to take pre-existing neurological co-morbidity into account, in her case small vessel disease (10) (see Table 1). A whole-body PET-MR revealed a high-uptake nodule at the accessory right parotid gland. An ultrasound scan of the neck showed a 1 cm salivary tumour in the right accessory parotid. Fine needle aspiration confirmed this to be a pleomorphic adenoma.
The workup for sarcoid was facilitated by the presence of an earlier CT scan. This had been done as part of the work-up for her appendiceal cancer. Two years ago, this CT scan had shown bilateral hilar lymph nodes and a calcified granuloma of the right lower lobe. The higher sensitivity of a chest CT compared to a chest x- ray for detecting granulomatous disease is well known (11). Having excluded alternative diagnoses, given the characteristic systemic findings from chest CT, elevated serum ACE, raised urinary calcium to creatinine ratio (0.83, upper limit of normal 0.79) and eosinophilia (12), plus her ocular phenotype of right vitreous haze on the OCT, optic disc swelling and neuropathy with thickened choroid, we made a clinical diagnosis of sarcoidosis.
A decision was made to start steroids, with a regime chosen by her admitting team: oral dexamethasone 8 mg bd for 2 days, 6 mg bd for 2 days, 4 mg bd for 2 days with gastric and bone protection. The day after starting steroids, her visual acuity had subjectively improved, and she no longer complained of pain. Follow up in the Eye Clinic confirmed an improvement in her right visual acuity, colour vision and visual fields; her right disc (Figures 7, 8) and macula (Figure 9) became less swollen, indicating resolution of vascular permeability. The hyperreflective spots seen previously have migrated vertically to pass the outer plexiform layer and were located within the inner nuclear layer (Figure 10). These spots give rise to a dense shadow cast over the underlying retinal layers. On IR (Infrared reflectance imaging) and fundoscopy this is perceived as macular striae or hard exudates. The left fundus remained normal.
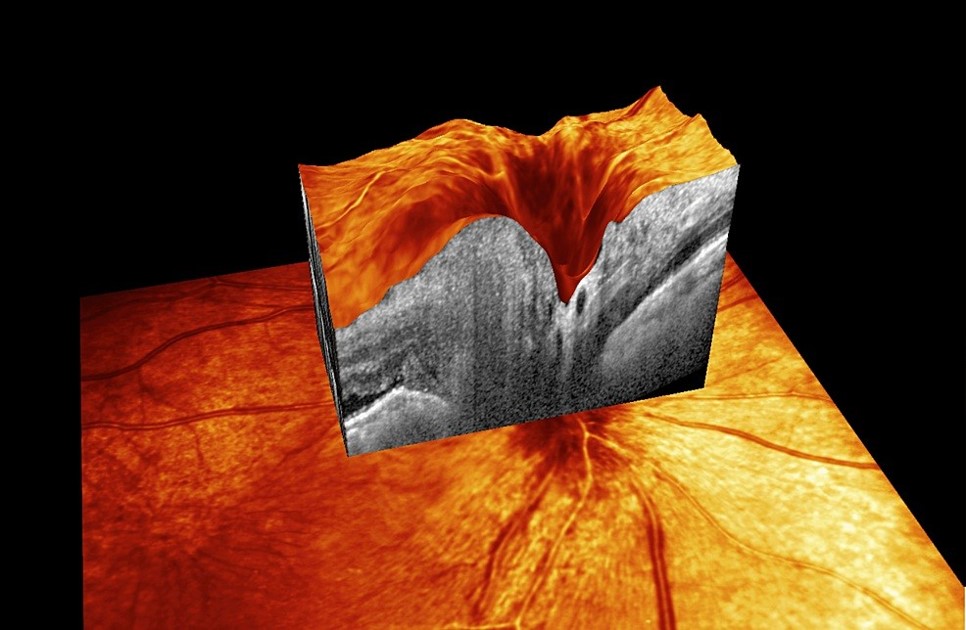
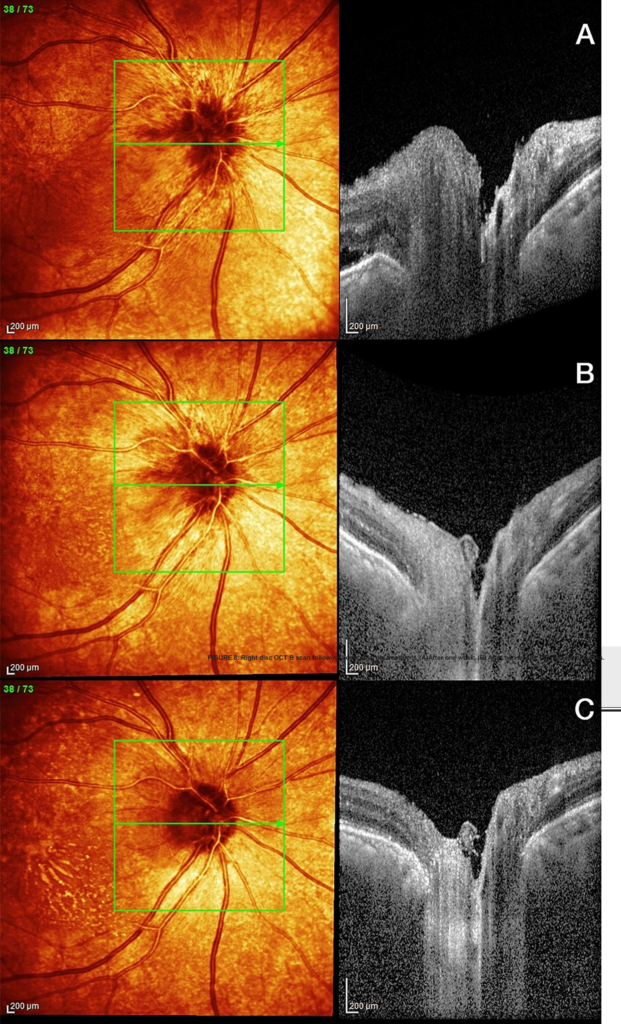
FIGURE 8: Right disc OCT B scan following corticosteroid treatment. (A) After one week, (B) After two weeks, (C): After four weeks.
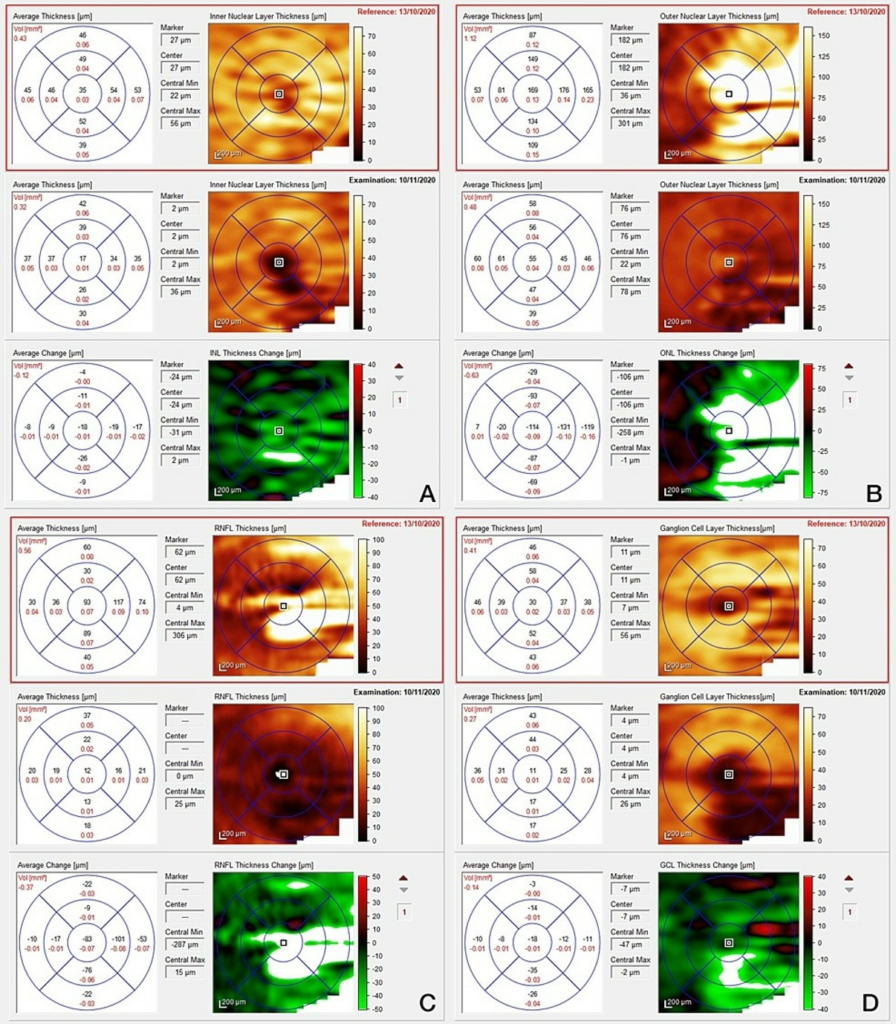
FIGURE 9: Optical coherence tomography of right macula following one week (top images) and one month (bottom images) of oral steroid treatment. (A) Inner nuclear layer, (B) Outer nuclear layer, (C) Macula ganglion cell layer, (D) Retina nerve fibre layer.
Following 1 month of treatment with prednisolone (40 mg/day), her right eye VA had improved to 6/12 (6/9 with pinhole), and left eye VA remained 6/6.
Discussion
The main novel finding of this case were longitudinal intraretinal signal changes suggestive of sarcoid. This case illustrates novel OCT features of intraretinal sarcoidosis: vertically migrating hyperreflective foci throughout the outer nuclear layer, extending to and including the outer plexiform layer. These migrating hyperreflective spots are different to the static hyperreflective spots located at vascular plexus adjacent to the upper and lower border of the inner nuclear layer seen due to a venous outflow problem in optic disc swelling.
There is disruption, irregularity and elevation of the photoreceptor layer and retinal pigment epithelium with significant subretinal fluid. Sattler’s medium-sized vessel choroidal layer in the right eye is enlarged, a finding previously identified as potentially helpful in distinguishing sarcoidosis from tuberculosis (13).
Vitreous activity is visible in the right eye; this was not apparent at the slit lamp examination as there was a nucleosclerotic and posterior subcapsular cataract and pupil dilation was limited due to pseudoexfoliation.
Following a review of the literature, we have identified two reports of sarcoidosis and intraretinal hyperreflective spots (in (14) and (5)). Similar to the present case, the Dutch patient (14) also had evidence of posterior uveitis. There are reports of disc oedema without anterior segment inflammation e.g., Pao et al (15) and of subretinal fluid without any hyperreflective foci e.g. Modi et al (16). The present case is unique in that we see OCT evidence of migration of these hyperreflective foci over time. The presence of hyperreflective foci has been noted in patients with age-related macular degeneration (17), vitelliform lesions (18) and diabetic macular oedema (19), and studies using immunostaining and confocal microscopy suggest they represent activated microglia which have traversed the retinal pigment epithelium.
Conclusions
Expanding on earlier cross-sectional observations, this case study demonstrates the utility of longitudinal spectral domain, high resolution retinal OCT to delineate the dynamics of compartmentalized ocular sarcoidosis. Treatment response may be monitored by resolution of disc oedema, migrating retinal hyper reflective spots, intraretinal and choroidal fluid and vitreous haze. Each of these pathologies has the risk of complications secondary to sarcoid or its treatment and given that this condition continues to present diagnostic challenges, a multi-disciplinary approach is recommended.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgements
We would like to thank Mr Serhan Miah for his help with fundus photography and OCT.
References
- Scadding JG. The eponymy of sarcoidosis. Journal of the Royal Society of Medicine [Internet]. 1981 Feb 1 [cited 2023 Aug 25];74(2):147–57. Available from: https://pubmed.ncbi.nlm.nih.gov/7009861/
- LEVITT JM. BOECK’S SARCOID WITH OCULAR LOCALIZATION: SURVEY OF THE LITERATURE AND REPORT OF A CASE. Archives of Ophthalmology [Internet]. 1941 Sep 1 [cited 2023 Aug 25];26(3):358–88. Available from: https://jamanetwork.com/journals/jamaophthalmology/article-abstract/616035
- Mochizuki M, Smith JR, Takase H, Kaburaki T, Acharya NR, Rao NA. Revised criteria of International Workshop on Ocular Sarcoidosis (IWOS) for the diagnosis of ocular sarcoidosis. British Journal of Ophthalmology. 2019 Feb 23;103(10):1418–22.
- Campagna G, Prospero Ponce CM, Vickers A, Hong BYB, Pellegrini F, Cirone D, et al. Neuro-Ophthalmic Sarcoidosis. Neuro-Ophthalmology. 2019 Feb 26;44(5):319–26.
- Levine ES, Luísa S.M. Mendonça, Baumal CR, Chin AT, Rifkin LM, Waheed NK. Choroidal nonperfusion on optical coherence tomography angiography in a case of unilateral posterior segment ocular sarcoidosis misdiagnosed as MEWDS. American Journal of Ophthalmology Case Reports. 2020 Dec 1;20:100944–4.
- Koestinger A, Guex-Crosier Y, Borruat FX . Autoimmune retinal dysfunction in sarcoid chorioretinopathy. Klinische Monatsblatter Fur Augenheilkunde [Internet]. 2006 May 1 [cited 2023 Aug 25];223(5):428–30. Available from: https://pubmed.ncbi.nlm.nih.gov/16705523/
- Koczman JJ, Rouleau J, Gaunt M, Kardon RH, Wall M, Lee AG. Neuro-ophthalmic Sarcoidosis: The University of Iowa Experience. 2008 May 1;23(3):157–68.
- Hata M, Miyamoto K. Causes and Prognosis of Unilateral and Bilateral Optic Disc Swelling. Neuro-Ophthalmology [Internet]. 2017 Apr 10;41(4):187–91. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5762143/
- Henderson AD, Tian J, Carey AR. Neuro-Ophthalmic Manifestations of Sarcoidosis. Journal of Neuro-Ophthalmology. 2020 Oct 26;41(4):e591–7.
- Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nature Reviews Neurology [Internet]. 2018 Aug 31;14(10):577–89. Available from: https://www.nature.com/articles/s41582-018-0058-z
- Levy A, Hamzeh N, Maier LA. Is it time to scrap Scadding and adopt computed tomography for initial evaluation of sarcoidosis? [Internet]. f1000research.com. 2018 [cited 2023 Aug 25]. Available from: https://f1000research.com/articles/7-600
- Renston JP, Goldman ES, Hsu RM, Tomashefski JF. Peripheral Blood Eosinophilia in Association With Sarcoidosis. Mayo Clinic Proceedings. 2000 Jun;75(6):586–90.
- Mehta H, Sim DA, Keane PA, Zarranz-Ventura J, Gallagher K, Egan CA, et al. Structural changes of the choroid in sarcoid- and tuberculosis-related granulomatous uveitis. Eye [Internet]. 2015 Aug 1 [cited 2023 Aug 25];29(8):1060–8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4541349/
- Burggraaff MC, Trieu J, de Vries-Knoppert WAEJ, Balk L, Petzold A. The Clinical Spectrum of Microcystic Macular Edema. Investigative Opthalmology & Visual Science. 2014 Feb 18;55(2):952.
- Pao Shu-I, Chang YH, Hsu CK, Lu DW. A rare manifestation of neuro-ophthalmic sarcoidosis: A case report. Taiwan Journal of Ophthalmology [Internet]. 2016 [cited 2023 Aug 25];6(1):45–51. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5602126/
- Modi YS, Epstein A, Bhaleeya S, Harbour JW, Albini T. Multimodal imaging of sarcoid choroidal granulomas. Journal of Ophthalmic Inflammation and Infection [Internet]. 2013 Aug 23 [cited 2023 Aug 25];3:58. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3765912/
- Coscas G, De Benedetto U, Coscas F, Li Calzi CI, Vismara S, Roudot-Thoraval F, et al. Hyperreflective Dots: A New Spectral-Domain Optical Coherence Tomography Entity for Follow-Up and Prognosis in Exudative Age-Related Macular Degeneration. Ophthalmologica. 2013;229(1):32–7.
- Chen KJ, Jung JJ, Curcio CA, Chandrakumar Balaratnasingam, Gallego-Pinazo R, Dolz-Marco R, et al. Intraretinal Hyperreflective Foci in Acquired Vitelliform Lesions of the Macula: Clinical and Histologic Study. 2016 Apr 1;164:89–98.
- Lee H, Jang H, Choi YA, Kim HC, Chung H. Association Between Soluble CD14 in the Aqueous Humor and Hyperreflective Foci on Optical Coherence Tomography in Patients With Diabetic Macular Edema. Investigative Opthalmology & Visual Science. 2018 Feb 1;59(2):715.
